Projects
Active Projects
Single-Cell Level, Feedback Controlled Electroporation (Open Project)

Electroporation is a widely used, safe, non-viral approach to deliver foreign vectors into many different cell types. When a cell is exposed to an electric field of the appropriate strength, the membrane undergoes reversible electrical breakdown, where transient pores form in the membrane, allowing molecular transport into the cell. The controlled intracellular delivery of biomolecules and therapeutics enables the ability to study and engineer fundamental cellular processes and has therefore been a major focus in biomedical research and clinical medicine. This work is a collaborative effort at Rutgers, involving 4 primary investigators (Jeffrey Zahn, David Shreiber, Hao Lin, Jerry Shan), with the primary focus of developing a microfluidic technology for performing controlled single-cell level transfection in a continuous, serial fashion. Recently published work showcases the electroporation platforms capability of electrically detecting the presence of a cell in the electroporation zone, applying a prescribed electric pulse of known strength, and measuring the degree of cell membrane permeabilization (both electrically and optically) in real time. Current work involves the validation of the platform for performing more clinically relevant transfections, such as plasmid DNA, with the ultimate goal of developing a platform for performing feedback controlled electroporation, that is independent of the cell-to-cell variability of a given population and across different cell types.
Sherba JJ, Atzampou M, Lin H, Shan JW, Shreiber DI, and Zahn JD. “The Fabrication and Operation of a Continuous Flow, Micro-Electroporation System with Permeabilization Detection.” Journal of Visualized Experiments. 2022, 179:e63103. https://www.jove.com/t/63103/the-fabrication-operation-continuous-flow-micro-electroporation
Sherba, JJ, Hogquist S, Lin H, Shan JW, Shreiber DI, and Zahn, JD. “The effects of electroporation buffer composition on cell viability and electro-transfection efficiency.” Nature Scientific Reports. 2020, 10(1):3053. https://www.nature.com/articles/s41598-020-59790-x https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7033148/
Zheng, M, Sherba, JJ, Shan HW, Lin H, Shreiber DI, Zahn JD. “Continuous-flow, electrically-triggered, single cell-level electroporation.” Technology. 2017, 5(1):31-41. https://www.worldscientific.com/doi/abs/10.1142/S2339547817500017
Engineering Neural Tissue Models
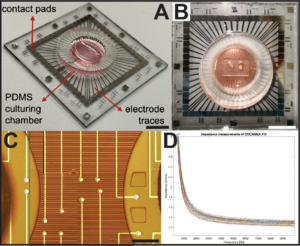
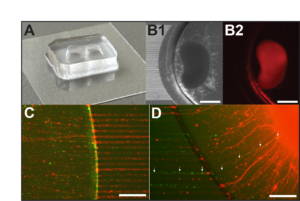
Existing therapies for autism spectrum disorders (ASDs) have had minimal clinical success due to our limited understanding about the underlying pathophysiology in the human brain. Evidence suggests that individuals with ASDs exhibit long-range connectivity deficits in the brain which directly impair information transfer between brain regions during key developmental stages. To date, there is limited reported work on in vitro platforms for modeling and assaying long-range axonal connectivity, a process vital for the integration of distal neural circuits in the human brain. This work combines human induced pluripotent stem cell (hiPSC)-derived cerebral organoid (CO) cultures and microfabrication bioengineering to recapitulate the long-range axonal projections formed between brain regions during human neurodevelopment. Using the fabricated compartmentalized platform, we demonstrated the formation of a functional connecting tract between CO pairs. Further, it enables investigation of long-range axonal connectivity dynamics using standard neuronal characterization techniques including calcium imaging, electrophysiological recordings, and immunohistochemistry. We hope that this work will provide a powerful model of disrupted connectivity underlying mental disorder pathophysiology for basic science and translational research, providing insights into potential treatments to restore neural pathway functionality and improve patient well-being.
Fantuzzo, JA, Robles DA, Mirabella VR, Hart RP, Pang ZP, and Zahn JD. “Development of a high-throughput arrayed neural circuitry platform using human induced neurons for drug screening applications.” Lab on a Chip. 2020, 20:1140-1152 https://pubs.rsc.org/en/content/articlelanding/2020/lc/c9lc01179j https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7339603/
Fantuzzo, JA, De Filippis L, McGowan H, Yang N, Ng YH, Halikere A, Liu JJ, Hart RP, Wernig M, Zahn JD, and Pang ZP. “μNeurocircuitry: establishing in vitro models of neurocircuits with human neurons.” Technology. 2017, 5(2):87-97. https://www.worldscientific.com/doi/abs/10.1142/S2339547817500054 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5541685/
Fantuzzo, JA, Mirabella VR, Hamod A, Hart RP, Zahn JD, and Pang ZP. “Intellicount: High-throughput quantification of synaptic protein puncta by machine learning.” eNeuro. 2017, 4(6): . https://www.eneuro.org/content/4/6/ENEURO.0219-17.2017 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5718246/
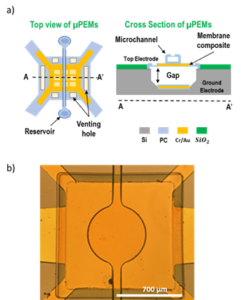
Development of Polymer Electrostatic Actuators with Integrated Microfluidics
This work is developing Micromachined Parylene-based Electroactive Membranes (μPEMs) with embedded microfluidic channels. These devices are formed as MEMS-based electrostatic actuator membranes over a bulk micromachined cavity, with microshell microfluidic architectures defined on top of the actuator. Monolithic integration of actuators with microfluidic components enables controllable mechanical cell manipulations for use in micromixers, particle manipulators, and applying strain to adherent cells cultured on top of the membrane to enable non-viral cell transfection and directed cell differentiation of mechanically sensitive stem cells for cellular engineering applications.
Rebolledo Uscanga, FA, Pierce, M, and Zahn, JD. “”Fabrication and development of novel micromachined parylene-based electroactive membranes with embedded microfluidic architectures.” Journal of Micromechanics and Microengineering. 2023, 33, 095010. https://iopscience.iop.org/article/10.1088/1361-6439/ace6b0
Other Projects
Gene Delivery by Applied Mechanical Stress
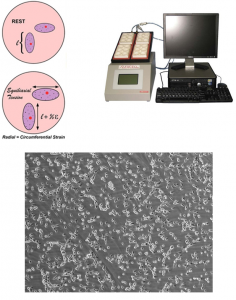
Gene transfection methods has been widely used to delivery negative charged nucleic acids (DNA and RNA) into cells with negative charged plasma membrane. Our research in this area focuses on optimization of novel efficient transfection method by applying mechanical stress/strain to cells by cyclic or static stretching of the cells in vitro. We are also investigating a modulation of mechanical stress on cells diverts different endocytic pathways in cells. In addition, ongoing studies also investigate a various endocytosis pathways involved in gene transfection by applying mechanical stress and identification of endocytosis pathways (such as CLIC/GEECs) that involved in DNA uptake.
Analysis of Blood Clot Rupture in a Microfluidic Device
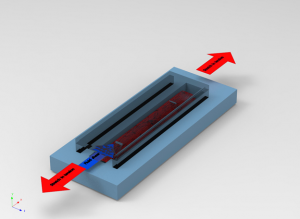
Blood clotting and biomechanics of blood clot rupture under fluid shear tension is a complex process. Our research in this area focuses on to develop a biomimetic microfluidic device to study blood clot rupture mechanics by stretching of blood clot in microfluidic device. We are also investigating a blood clot rupture under stretch and tension of fluid to replicate biomimetic conditions as blood clot rupture in artery under shear tension by blood flow.
Study of Network Hemodynamics in an Artificial Microvasculature
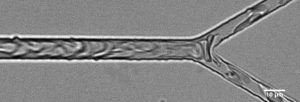
Consisting of the smallest blood vessels, microvascular networks are responsible for gas and nutrient transfer as well as the regulation of blood flow to the bodies tissues. When the function of the microcirculation is hindered, major health issues, even death can occur. In the microvasculature blood can no be treated as a bulk fluid and behaves as a suspension of highly deformable particles, predominately red blood cells (RBCS) suspended in plasma. The networks are characterized by bifurcations and convergences, the former leading to heterogenous distribution of RBCs throughout the network and in some extreme cases can result in RBC free vessels. Using microfabricated networks as we aim to study network hemodynamics and RBC based dynamics of blood flow in simple bifurcations and microvascular networks analyzing the effects of feeder channel hematocrit, Q*, and network topology.
Pskowski, A, Bagchi, P, and Zahn, JD. “Hematocrit skewness along sequential bifurcations within a microfluidic network induces significant changes in downstream red blood cell partitioning.” Biomicrofluidics. 2022, 16(6):064104. https://pubs.aip.org/aip/bmf/article-abstract/16/6/064104/2835516/Hematocrit-skewness-along-sequential-bifurcations
Pskowski A, Bagchi P, and Zahn JD. “Investigation of red blood cell partitioning in an in vitro microvascular bifurcation.” Artificial Organs. 2021, 45(9):1083-1096. https://onlinelibrary.wiley.com/doi/10.1111/aor.13941