Research
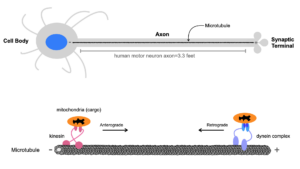
We aim to understanding the regulation of intracellular transport within highly polarized cells. We study axonal transport regulation to discover novel pathways that play an important role in intracellular transport and neuronal function.
Intracellular transport is the transport of mRNAs, proteins, and numerous cellular organelles by molecular motor proteins. This transport is important in all cells for cellular function. However, phenotypes from impairment of intracellular transport are more pronounced in neurons because of their highly polarized cellular morphologies. For instance, mutations in the microtubule motor protein cytoplasmic dynein are linked to neurological disorders including spinal muscular atrophy with lower extremity predominance (SMA-LED) and malformations in cortical development (MCD), while mutations within Kinesin-1 are linked to neurological disorders including autosomal dominant spastic paraplegia type 10 (SPG10) and amyotrophic lateral sclerosis (ALS).
Within the axon microtubules are oriented with their minus-ends toward the cell body and plus-ends toward the synaptic terminal. Plus-end directed kinesins transport cargo from the cell body to the synaptic terminal, while cytoplasmic dynein transports cargo along microtubules from the synaptic terminal to the cell body.
Research Approaches
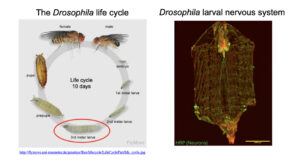
We use the model organism, Drosophila melanogaster or the fruit fly as our primary research system. Drosophila is an excellent model organism for our studies. 60% of the protein coding genes are conserved between flies and humans enabling mechanistic studies in flies that relevant to understanding the function of these proteins in humans. Drosophila is a genetically tractable system and the Drosophila research community has developed numerous tools to study the function of gene products. In addition, the life cycle of Drosophila is short, going from egg to adult in just 9-10 days. Importantly for our research, the motor neurons are easily accessible for live imaging organellar transport in an intact nervous system.
We use genetics, biochemical approaches, and microscopy techniques in our studies.
STRIPAK
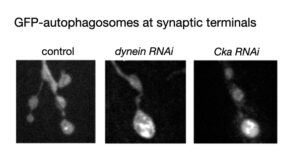
Dr. Neisch carried out a genetic screen to identify proteins conserved from flies to humans, that when depleted resulted in accumulation of a dynein specific cargo, autophagosomes, at synaptic terminals. This screen led to the identification of the STRIPAK (STRiatin-Interacting Phosphatase and Kinase) complex as a regulator of intracellular transport of a subset of organelles. STRIPAK is a highly evolutionarily conserved (from fungi to humans) complex that contains kinases and the phosphatase PP2A. We showed that core-components of the complex (Cka, Strip, Mob4) were required for transport regulation, and that PP2A phosphatase activity within the complex was also required for transport regulation.

To better understand STRIPAK intracellular transport regulation, we are currently addressing the following questions regarding the complex:
- How do core components of the STRIPAK complex, molecularly function in the complex to regulated axonal transport?
- Are the core components themselves regulated and is there differential regulation in different regions of the neuron?
- Does neuronal activity modulate STRIPAK mediated transport?
- What are the STRIPAK phospho-substrate(s) important in axonal transport regulation?
Additional studies
We are also continuing our efforts to understand signaling pathways and proteins that modulate the dynein complex to influence axonal transport, using genetic and biochemical approaches.