Integrated Modeling & Control of Continuous Downstream Pharmaceutical Processes
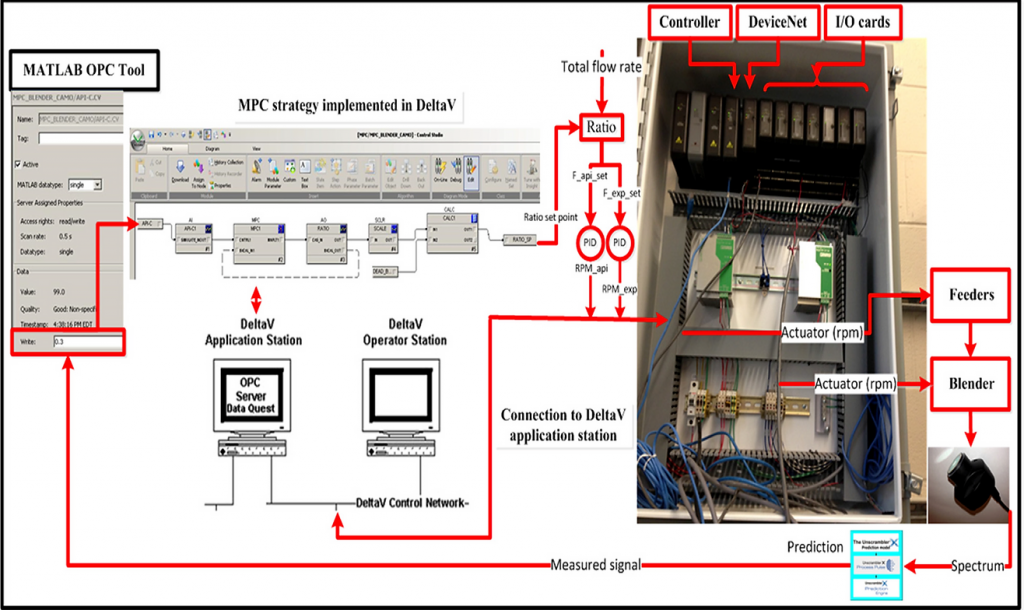
As continuous manufacturing of pharmaceutical processes continues gather importance from a scientific and technological perspective, we are working to develop integrated open-loop and closed-loop flowsheet configurations of the downstream pharmaceutical process from crystallization to tablet dissolution. This would enable us to better understand from a process-wide perspective the effects of upstream variables on downstream product attributes. We are also studying the effect of different control strategies (structural and parametric) to maximize the performance of the integrated process, both in simulation and in the pilot-plant. The integration of in-line sensing tools is a key aspect of closed-loop control of the pilot-plant.
Real Time Monitoring

Hybrid MPC-PID Control
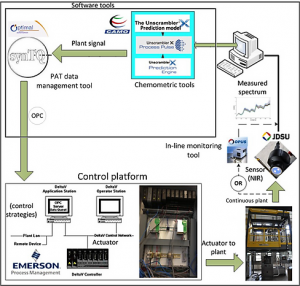
Hybrid Control
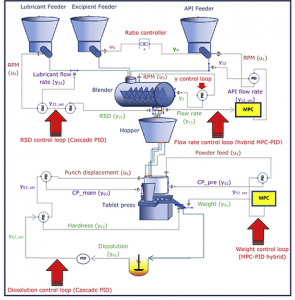
Control Scheme of Rutgers University Continuous Pharmaceutical Production Line
-
- R. Singh, A. Roman, R. Romanach, M. Ierapetritou, R. Ramachandran. Real time monitoring of blend density for coupled feed-forward/feed-back control of a continuous direct compaction tablet manufacturing process. International Journal of Pharmaceutics, 495, 612-625, 2015.
- R. Singh, F. Muzzio, M. Ierapetritou, R. Ramachandran. A combined feed-forward/feed-back control system for a QbD based continuous tablet manufacturing process. Processes, 3(2), 339-356, 2015.
- R. Singh, F.J. Muzzio, M. G. Ierapetritou, R. Ramachandran. Plant-wide control of a continuous tablet manufacturing process for Quality-by-Design based pharmaceutical manufacturing. Computer Aided Chemical Engineering, 37, 2183-2188, 2015.
- R. Singh, M. Sen, M. Ierapetritou, R. Ramachandran. Integrated moving horizon based real time optimization and hybrid MPC-PID control of a direct compaction continuous tablet manufacturing process. Journal of Pharmaceutical Innovation, 1-21, 2015.
- R. Singh, A. Sahay, K.M. Karry, F. Muzzio, M. Ierapetritou, R. Ramachandran. Implementation of an advanced hybrid MPC-PID control system using PAT tools into a direct compaction continuous pharmaceutical tablet manufacturing pilot plant. International Journal of Pharmaceutics, 473, (1-2), 38-54, 2014.
- M. Sen, R. Singh, R. Ramachandran. A hybrid MPC-PID control system design for the continuous purification and processing of active pharmaceutical ingredients, Processes, 2(2), 392-418, 2014.
- R. Singh, A. Sahay. F.J. Muzzio, M.G. Ierapetritou, R. Ramachandran. A Systematic framework for onsite design and implementation of a control system in a continuous tablet manufacturing process. Computers & Chemical Engineering, 66, 186-200, 2014.
- M. Sen, R. Singh, R. Ramachandran. Simulation based design of an efficient control system for the continuous purification and processing of active pharmaceutical ingredients, Journal of Pharmaceutical Innovation, 9 (1), 65-81, 2014.
- R. Singh, D. Barrasso, A. Chaudhury, M. Sen, M. Ierapetritou, R. Ramachandran. Closed-loop feedback control of a continuous pharmaceutical tablet manufacturing process via wet granulation. Journal of Pharmaceutical Innovation, 9 (1), 16-37 , 2014.
- R. Singh, M. Ierapetritou, R. Ramachandran. Hybrid advanced control of flexible multipurpose continuous tablet manufacturing process via direct compaction, Computer Aided Chemical Engineering, 32, 757-762, 2013.
- M. Sen, A. Rogers, R. Singh, A. Chaudhury, J. John, M. Ierapetritou and R. Ramachandran. Flowsheet modeling and optimization of an integrated pharmaceutical purification-processing manufacturing operation. Chemical Engineering Science, 102, 56-66, 2013.
- M. Sen, A. Chaudhury, J. John, R. Singh, R. Ramachandran. Multi-scale flowsheet simulation of an integrated continuous purification-downstream pharmaceutical manufacturing process. International Journal of Pharmaceutics, 445 (1-2), 29-38, 2013.
- F. Boukouvala, A. Chaudhury, M. Sen, R. Zhou, L. Mideskowski, M .Ierapetritou and R. Ramachandran. Computer-Aided Flowsheet Simulation of a Continuous Tablet Manufacturing Process incorporating Wet Granulation, Journal of Pharmaceutical Innovation, 8 (1), 11-27, 2013.
- R. Singh, M. Ierapetritou and R. Ramachandran. System-wide hybrid MPC-PID control of a continuous pharmaceutical tablet manufacturing process via direct compaction. European Journal of Pharmaceutics & Biopharmaceutics, 85(3B), 1164-1182, 2013.
- R. Singh, M. Ierapetritou and R. Ramachandran. An engineering study on the enhanced control and operation of continuous manufacturing of pharmaceutical tablets via roller compaction. International Journal of Pharmaceutics, 307-326, 438, 2012.
- F. Boukouvala, V. Niotis, R. Ramachandran, F. J. Muzzio and M. I. Ierapetritou. An integrated approach to dynamic flowsheet modeling of a continuous tablet manufacturing process. Computers & Chemical Engineering,42, 30-47, 2012.
- R. Ramachandran, J. Arjunan, A. Chaudhury and M. Ierapetritou. Model-based control-loop performance assessment of a continuous direct compaction pharmaceutical process. Journal of Pharmaceutical Innovation, 6, 249-263, 2011.
- S. Schaber, D. Gerogiorgis, R. Ramachandran, J.M.B. Evans, P.I. Barton and B.L. Trout. Economic Analysis of Integrated Continuous and Batch Pharmaceutical Manufacturing: A Case Study. Industrial Engineering Research & Chemistry, 50, 10083-10092, 2011.
- F. Boukouvala, R. Ramachandran, A. Vanarase, F.J. Muzzio and M.G. Ierapetritou. Computer Aided Design and Analysis of Continuous Pharmaceutical Manufacturing Processes. Computer-Aided Chemical Engineering, 29, 216-220, 2011.