Percutaneous Penetration Modifiers and Formulation Effects
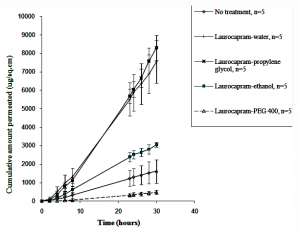
This study aimed at investigating the effect of vehicle selection on the enhancement effects of several “chemical enhancers”. The enhancement/retardation of percutaneous permeation of diethyl-m-toluamide (DEET) in presence of five percutaneous penetration modifiers (laurocapram, 3-dodecanoyloxazolidin-2-one (N-0915), S,S-dimethyl-N-(4-bromobenzoyl) iminosulfurane (DMBIS), S,S-dimethyl-N-(2-methoxycarbonylbenzenesulfonyl) iminosulfurane (DMMCBI) and tert-butyl 1–dodecyl-2-oxoazepan-3-yl-carbamate (TBDOC)) was investigated. These permeation modifiers were formulated in water, propylene glycol (PG), ethanol or polyethylene glycol 400 (PEG 400). The permeation studies indicated that laurocapram enhanced DEET permeation in PG, but retarded in PEG 400. Likewise, N-0915 acted as a retardant in ethanol and PEG 400, but not in water. DMBIS decreased the permeation in ethanol as compared to permeation in water, PEG 400 or PG. Similarly, DMMCBI acted as a retardant with ethanol and PEG 400, but not with water or PG. TBDOC revealed its activity as a retardant with ethanol, but behaved as an enhancer with water, PG and PEG 400. In addition, penetration modifier interactions with stratum corneum ceramides were investigated using chemical modeling.
This investigation is significant since it confirms the role of pharmaceutical formulations and shows for the first time that an enhancer can become a retardant or vice versa depending upon the vehicle in which it is applied to the skin. Hence, we should be using the term “penetration modifiers” rather than “chemical enhancers” for all such compounds.