Research
Two-Component Systems
Two-Component Systems (TCSs) are a central theme in our research projects. In most bacteria, several dozen TCSs provide adaptive responses to environmental stimuli, regulating processes such as nutrient utilization, swimming behavior, biofilm formation, sporulation, or virulence factor expression. TCS signaling utilizes a core phosphotransfer pathway between two conserved components. In canonical TCSs, a histidine kinase that is regulated by environmental stimuli autophosphorylates at a histidine residue, creating a high-energy phosphoryl group that is subsequently transferred to an aspartate residue in a response regulator protein, promoting an active conformation that elicits a response. Variable input domains in histidine kinases and variable output domains in response regulators provide a versatile scheme that can be used to couple almost any physical or chemical stimuli to any output response.
Regulation of Polysaccharide Utilization in Bacteroides
Dietary fiber is an important carbon source for some of the most abundant species of the gut microbiota. In Bacteroidetes, gene clusters encoding protein systems necessary for metabolism of specific complex carbohydrates are colocalized into polysaccharide utilization loci, or PULs. Bacteroides thetaiotaomicron encodes 88 different PULs and their transcriptional expression is strictly regulated. In B. theta 32 PULs are regulated by an unusual class of TCSs known as Hybrid Two-Component Systems (HTCSs). In HTCSs, the transmembrane sensor histidine kinase and response regulator transcription factor of canonical TCSs are fused into a single polypeptide chain with extracellular ligand sensing, histidine autophosphorylation, phosphoryl transfer, and transcriptional regulation occurring within a single transmembrane dimer. Our studies focus on mechanistic characterization of phosphorylation and DNA binding of representative HTCS proteins. Structural characterization is being pursued by X-ray crystallography and cryo-EM. Cellular studies are aimed at defining the regulons of individual HTCSs and the coordination between HTCSs that determines preferential utilization of different dietary polysaccharides and host glycans.

Chemotaxis Signaling in a Nitrogen Fixing Bacterium

This collaborative project with Birgit Scharf at Virginia Tech focuses on chemotactic signaling in Sinorhizobium meliloti, a bacterium that fixes nitrogen, converting atmospheric nitrogen to a biologically usable form. Rhizobia are a unique group of motile soil bacteria that can engage in specific symbiotic relationships with leguminous plants such as peas, soy beans and alfalfa. This symbiosis supplies the host plant with nitrogen, which is the most limiting nutrient for plant growth. Chemotaxis systems, present in all motile bacteria, enable cells to move toward beneficial chemicals and away from harmful compounds. In rhizobia, chemotaxis allows cells to recognize and move toward plant hosts. Rhizobia use multiple chemoreceptor proteins to sense a wide spectrum of chemical compounds secreted by host plants. However, little is known about the specific processes involved in the regulation of sensitivity and adaptation, which are essential features for an effective chemotactic response. The overarching goal of this project is to decode the pathway that controls adaptation to stimuli. The specific project focuses on CheT, a novel component of chemotaxis systems in rhizobia. Studies in our lab use biochemical and structural approaches to define the interactions of CheT with other chemotaxis proteins, while studies in the Scharf lab aim to characterize the biochemical activity of CheT and determine its contribution to chemotactic behavior.
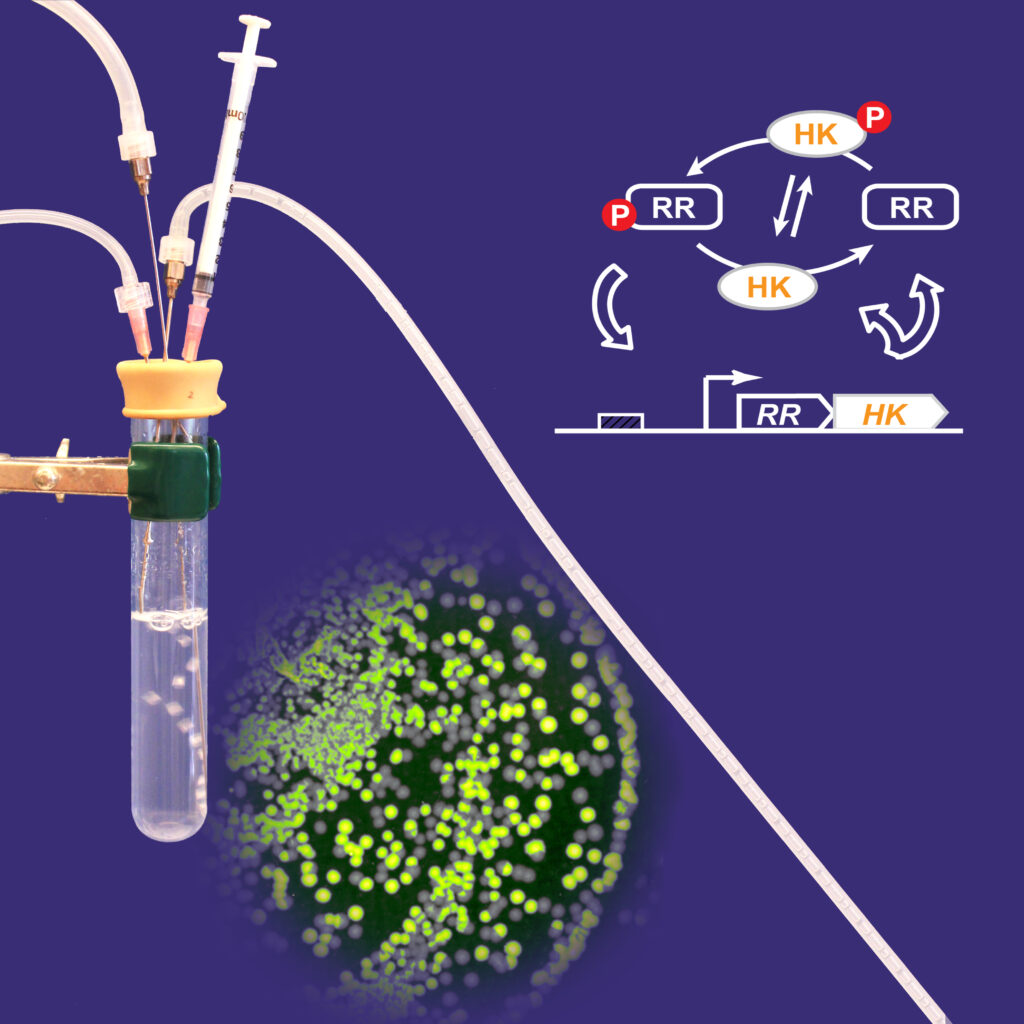
Two-Component System Design Principles
While structure and function of core elements are conserved, TCSs display enormous diversity. Data acquired from studies of many individual systems as well as global analyses have revealed differences in the magnitudes of enzyme activities, affinities of macromolecular interactions, concentrations of signaling proteins and system architectures that contribute to tuning response behavior to the needs of individual systems. We aim to understand design principles of TCSs to the extent that system behavior can be predicted, or at least rationalized, with knowledge of system parameters. We have used a combination of in vitro biochemical and cellular assays together with mathematical modeling to explore features that control system behavior in TCSs that regulate gene expression. Positive autoregulation, which increases levels of signaling proteins when systems are activated has been found to be optimized for fitness, graded responses to stimuli, regulation of timing and kinetics of gene expression and suppression of bistability. Cellular concentrations of many transcription factors are not in great excess relative to the numbers of their binding sites, suggesting that they might be sensitive targets for antimicrobial drug development.
Funding
Our laboratory gratefully acknowledges support from the NIH, NSF, and the Kronthal Family Foundation.

