Current Research
-
Development and evaluation of desoximetasone loaded non-ionic surfactant based novel dermal drug delivery using quality by design (QbD) approach.
Study Participant:
 Parin Shah
Parin ShahStudy Aims:
- To develop a niosomal vesicle based delivery system to deliver a variety of hydrophobic/hydrophilic drugs with improved bioavailability and stability.
- To develop a drug loaded noisome-containing topical gel for the treatment of various skin conditions such as psoriasis, eczema, itching, redness, etc. using sustained drug delivery.
- To produce an advanced way to deliver target specific drugs by improving drug availability at the site of action. Controlled release drug delivery helps to release drug over longer periods which reduces drug application frequency and/or reduces drug concentrations in the formulation.
The research work focused on the systematic development of non-ionic surfactant based topical drug delivery developed by advanced Quality by Design (QbD) approach. The research was subdivided into three phases. In the first phase, niosomes were prepared by considering various critical material attributes (CMAs) and critical process parameters (CPPs). Method of preparation, drug concentration, surfactant concentration, cholesterol concentration, and type of lipids were considered as CMAs. Systematically designed experiments were performed by changing drug, surfactant, and cholesterol concentrations to evaluate the impact of the change in raw materials on the final product. Mixing parameters (speed and time), internal and external phase volume, external phase temperature, and organic phase addition rate were considered as CPPs. Various formulations were manufactured by changing process parameters to evaluate their impact on the final product. Each formulation was evaluated for entrapment efficiency, particle size, polydispersity index (PDI), and zeta potential. The results showed that surfactant and cholesterol concentration, mixing parameters (time and speed), and organic phase addition rate shows recognize the impact on the niosome formulations.
In the second phase of the study, impacted parameters from the first phase were exhaustively studied by 25 full factorial design using JMP® statistical software. Experiments were performed as per the formulation combination proposed by the design of the experiment and evaluated for entrapment efficiency, particle size, polydispersity index (PDI), and zeta potential. All the results were thoroughly assessed. All the data were added into the JMP study design, and the profile predictor was generated based on the experimental data. The ideal formulation profile was predicted using profile predictor features from JMP statistical software. The suggested ideal formulation was manufactured to verify checkpoint, and obtained results were in-line with predicted results, which represent an accuracy of design of experiments (DoE) study. An additional batch was manufactured by changing the grade of surfactant to verify surfactant (principal component) chemistry impact on the niosome characteristics.
During the final phase of the study, topical gels were manufactured with niosomal dispersion using various synthetic or natural gelling agents. Different topical gels were manufactured by changing the concentration or grade of the carbomer or change in the gelling agent. Various gelling agents were used in identical concentrations to see the impact of the material on the final product, where carbomer 980 was the targeted gelling agent as it was utilized by the reference listed drug (RLD) marketed product formulation. Various concentrations of carbomer 980 were evaluated to match the gel physical characteristics with the reference product. Topical gels were evaluated for chemistry (assay, content uniformity, pH), physical parameters (yield stress, viscosity, rheology, flow curve, specific gravity, spreadability), and organoleptic properties.
Finally, studies related to anti-inflammatory glucocorticoid delivery in topical gel dosage form along with the impact of niosomes microstructure were conducted. The anti-inflammatory glucocorticoid drug, desoximetasone, was used as the model compound. Topicort® Gel (desoximetasone) 0.05% market product was used as the reference for the comparison of the niosome based topical gel product. Based on the evaluation, results showed that topical gel formulations with 0.7% Carbomer 980 were complete concordance with the reference product. The results also show that the rheological testing provided additional advantages over conventional methods for being sensitive, accurate, and versatile. Additionally, permeation experiments on human cadaver skin were conducted for comparison between niosome containing topical gel and the reference product. Dermal delivery of desoximetasone in the niosome containing topical gel formulation was found to be slower in comparison with the reference product. The result also showed a statistically significant increase in the amount of drug retained in the skin in the niosome containing topical gel formulation, which showed a sustained drug release pattern. The stability study of the niosomal dispersion and niosome containing topical gel shows that the niosome vesicle dispersion and topical gel products were stable at room temperature (25°C) and accelerated temperature (40°C) conditions with no significant changes in the on the final product. Results from the present study highlight that optimized ideal niosome formulations can efficiently deliver a hydrophilic/hydrophobic drug to the target site with improved stability. Also, the niosomal topical gel was able to have a reduced drug concentration and dose frequency, and this was achieved by controlling drug release from the niosome matrix to gain maximum therapeutic effectiveness.


-
APPLICATION OF SOLUBILITY-PHYSICOCHEMICAL-THERMODYNAMIC (SPT) THEORY FOR DESIGNING A TOPICALLY APPLIED THYMOQUINONE POLYMER FILM TO TREAT INFECTED WOUNDS
Study Participants: Anika Haq, Yong Mao (Chemistry) and Suneel Kumar PhD (Biomedical Engineering)
Selection of enhancers using FFE (Formulating for EfficacyTM – ACT Solutions Corp.) software
During her PhD studies, Anika found that the overall mechanism of action of skin penetration enhancers is best explained by the Solubility-Physicochemical-Thermodynamic (SPT) theory. The SPT theory puts forward the concept that the mode of action of enhancers is related to solubility parameters, physicochemical interactions and thermodynamic activity. She used experimentally derived permeation data, various physicochemical and solubility parameters (ingredient active gap (IAG), ingredient skin gap (ISG), solubility of active in the formulation (SolV) and the formulation solubility in the skin (SolS)) generated by using FFE (Formulating for EfficacyTM – ACT Solutions Corp) software. The data suggests that there is an inverse relationship between measured flux and IAG values given that there is an optimum ingredient skin gap, SolV and SolS ratio. The study demonstrated that the flux is actually proportional to a gradient of thermodynamic activity rather than the concentration and maximum skin penetration and deposition can be achieved when the drug is at its highest thermodynamic activity. Anika’s work connected the solubility and physicochemical properties of the active and enhancers/ingredients with the thermodynamic activity of the model drug used in order to explain the mode of action of enhancers in a given formulation with a specific drug.
Anika used the enhancer selections obtained from the FFE (Formulating for EfficacyTM – ACT Solutions Corp) software the for the next part of her studies.
Design and testing of a novel thymoquinone transdermal drug delivery system
Thymoquinone (TQ) (2- isopropyl- 5- methyl- 1,4- benzoquinone) is the main constituent of Nigella sativa (Black cumin) seeds. Black seed contains up to 30-48% of TQ along with 15 amino acids, proteins, carbohydrates, fixed oils, volatile oils, alkaloids, saponins, crude fiber, minerals such as, calcium, iron, sodium and potassium. TQ is a yellow crystalline molecule and has a basic quinone structure. TQ has many pharmacological properties such as anticancer, anti-inflammatory, antioxidant, antiasthmatic and immunomodulatory effects.
The ability to formulate TQ is hindered by various factors. It is a lipophilic molecule (log P = 2.54) and is poorly soluble in aqueous media that results in bioavailability issues. Additionally, its thermo-labile nature limits the application of nano-formulation techniques to enhance its bioavailability. On the other hand, the compound’s low molecular weight (164.2 gmol-1), low melting point (44-45°C), and lipophilicity can be useful for incorporation into a topical or transdermal delivery system.
Anika studied the effect of an ethanol and propylene glycol solvent system for the transdermal delivery of thymoquinone (TQ). The effects of penetration enhancers on the in vitro skin permeation and TQ skin absorption were studied using human cadaver skin in Franz diffusion cells. The permeation of saturated solutions of TQ was investigated with 5% v/v of each of the following enhancers: Azone (laurocapram), Transcutol® P (Tc), oleic acid, ethanol, Polysorbate 80 (Tween 80), and N-methyl-pyrrolidone (NMP). The data suggested that Azone, oleic acid and Tc were able to provide adequate TQ flux and may be the agents of choice for use in a novel transdermal formulation of TQ. These penetration enhancers were also able to generate TQ reservoirs in the skin that may be useful to provide sustained release of TQ from the stratum corneum over longer periods of time. The study also demonstrated “pull” or “drag” effect of permeation enhancers and vehicle on TQ skin deposition. These studies suggested that ethanol was able to “pull” more drug into the skin and all the enhancers used in this study showed some “pulling” effect. Finally, she designed and characterized a biocompatible novel topical polymeric film system that had the potential to deliver antibacterial/anti-inflammatory agent thymoquinone (TQ) directly to the skin target site and that may be useful for the treatment and management of wound infections. The polyvinyl pyrrolidone (PVP) matrix-type films containing TQ were prepared by the solvent casting method using dibutyl phthalate as a plasticizer and Azone (laurocapram) as a penetration enhancer. The developed films were evaluated for thickness, drug content uniformity, weight variation, flatness, folding endurance, percentage of moisture content and uptake which were found to 1.17 ± 0.04 mm, 100 ± 6.4 %, 82.04 ± 1.9 mg, 100%, 68 ± 2.38, 14.12 ± 0.42 %, and 2.26 ± 0.47 % respectively. In vitro skin permeation studies on human cadaver skin produced a mean flux of 2.3 µg/cm2/h. In vitro scratch assay results revealed that 100 ng of TQ had significant wound closure activity in human dermal fibroblast cells compared to both control (p = 0.0014) and positive control (p = 0.0004). Using human keratinocyte cell line, 100 ng TQ group showed 85% wound closure activity at day six which was significantly higher (p = 0.0001) than the control group. In a zone-of-inhibition (ZOI) assay, the presence of TQ-containing films completely wiped out Staphylococcus aureus in a 10 cm in diameter TSA (Tryptone soya agar) plates while 500 ug/mL gentamicin containing filters gave 10 mm of ZOI. In an ex vivo model, the presence of TQ-film eradicated the bacterial colonization on human cadaver skin. Furthermore, in the BALB/c mice wound model, TQ-films showed significant activity in controlling Staphylococcus aureus infection and promoting wound closure compared to control film. These results indicate, TQ/PVP films developed in this study have potential for the treatment and management of wound infection.
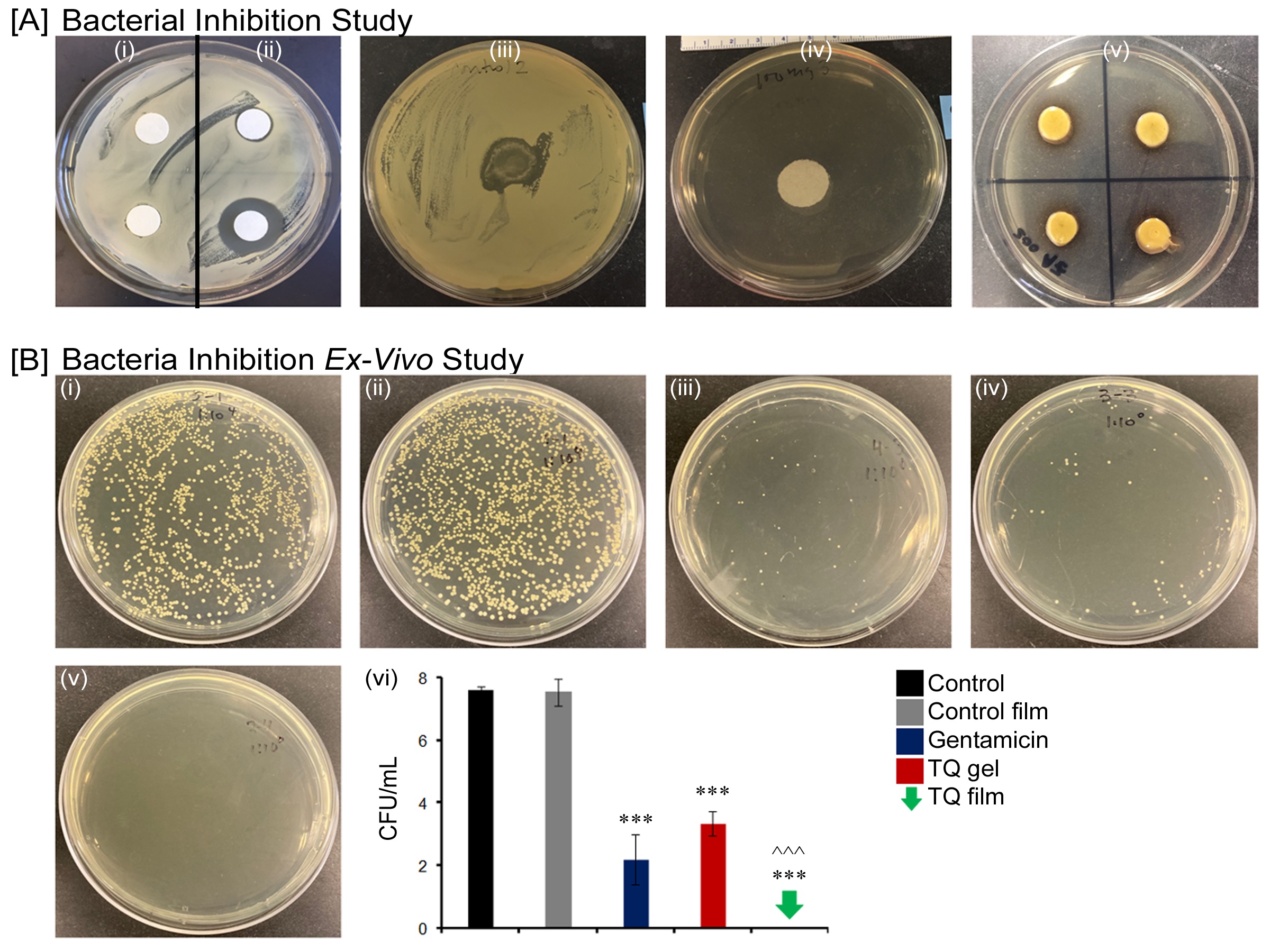
Bacterial inhibition study (A) Inhibition of bacterial growth on agar plate by Control negative (i); Gentamicin positive control 50 µg/mL (ii) right upper and 500 µg/mL (ii) right lower; Control film (iii); TQ gel (iv) and TQ film (v) against Staphylococcus aureus; (B) Ex vivo antibacterial activity by Control (i); Control film (ii); Gentamicin marketed cream (iii); TQ gel (iv); TQ film (v) and Log of bacterial reduction with different treatment groups. Data represent mean ± SD of four replicates. ***p = < 0.001 and ^^^p = < 0.05.
-
THE TRANSDERMAL ROUTE AS AN ALTERNATIVE: THE DELIVERY OF DRUGS FOR NEURODEGENERATIVE DISEASES
Study Participant: DINA WADIE AMEEN
The transdermal route is an attractive alternative for drug delivery with several advantages including avoidance of gastrointestinal side effects/ metabolism and hepatic first-pass effect, constant drug plasma level, being non-invasive, acting as a visual reminder of drug administration, and improved adherence to treatment. The latter is important for the successful management of neurodegenerative diseases, due to their chronic progressive nature requiring prolonged treatment. Despite the advantages, skin is a tough barrier for drug absorption and not many drugs can passively diffuse through the skin into the blood in amounts sufficient to exert a therapeutic effect. The objective of this research is to explore the feasibility and develop transdermal drug delivery systems of drugs for Alzheimer’s disease (AD) and multiple sclerosis (MS). Alzheimer’s disease (AD) is a progressive degenerative disorder affecting the brain function in the elderly. However, people younger than 55 years of age can also suffer from early onset, or familial AD due to inherited genetic mutations. The disease symptoms include memory loss, confusion, depression, hallucinations and related psychoses. AD is characterized by synaptotoxicity, neurotransmitter disturbances, and accumulation of two proteinaceous materials, namely amyloid-β (Aβ) plaques in the brain interstitium and tau tangles within the neurons. These events cause interference with the normal nutrition and function of the neurons leading eventually to their death and brain atrophy in later stages. AD represents a major public health problem in modern times. In 2020, it is estimated that there are about 5.8 million 65 years and older AD patients in the USA. By 2050 the number is expected to reach 13.8 million with the current situation of the lack of cure for this disease. It poses a huge socioeconomic burden due to the long duration of the disease and need for institutionalization or caregiving. In 2019, caregivers of AD patient provided an estimated 18.6 billion hours of unpaid assistance, a contribution to the nation valued at $244 billion.
In this work, we investigated the development of a transdermal drug delivery system containing galantamine, an oral drug for AD, in a drug-in-adhesive type of system. Different pressure sensitive adhesives, penetration enhancers, and drug loadings were tested to optimize the drug delivery system through permeation studies using Franz diffusion cells with human cadaver skin, and release and rheological studies. The optimized formulation had a flux enhancement ratio of 2.7-fold and was predicted to achieve a therapeutic plasma level using 20 cm² patch. The work also investigated the feasibility of transdermal delivery of dimethyl fumarate (DMF), an oral MS drug, by studying the effect of different penetration enhancers at varying concentrations on DMF permeation using vertical Franz diffusion cells and human cadaver skin. The most effective penetration enhancer was found to be 5% cineole with a 5.3-fold increase in enhancement ratio suggesting that DMF is a potential candidate for transdermal drug delivery. Additionally, the feasibility of transdermal co-delivery of DMF and nicotine as a potential treatment for AD was investigated through studying the effect of pH and nicotine form (free base vs. salt) on the permeability of both drugs using human cadaver skin and vertical Franz diffusion cells. The results suggested the possibility of interplay between pH, and ion-pair formation influencing the permeation of DMF if combined with an ionizable molecule (nicotine). Finally, the formulation of DMF as nanostructured lipid carriers (NLCs) was explored through investigating several methods of preparation and compositions and their effects on drug loading and entrapment efficiency. The study involved measuring particle size and distribution, NLCs morphology, drug release and permeation enhancement effect of NLC formulation. Microemulsion method was shown to be successful in producing DMF NLCs with acceptable characteristics and good penetration properties. In conclusion, using chemical penetration enhancers and formulation optimization approaches provide feasible approaches to develop transdermal drug delivery systems of the studied drugs for the treatment of AD and MS.
-
 Systematic development of nanocapsule based drug delivery using by Quality by Design (QbD) methodologies
Systematic development of nanocapsule based drug delivery using by Quality by Design (QbD) methodologiesStudy Participant: Ben Goodyear
The research work focused on the systematic development of nanocapsule based drug delivery using by Quality by Design (QbD) methodologies. Research will be subdivided into three phases. In the first phase, nanocapsules will be manufactured lab bench scale to measure impacting variables with the greatest impact on nanocapsule critical quality attributes.
Critical material attributes important for developing nanocapsules are polymer concentration (PCL mg/mL), and surfactant type (non-ionic), & surfactant concentration (mg/mL). Materials are systematically selected for optimization and used to create nanocapsules using a common manufacturing method known as the solvent evaporation nano-precipitation method.
Critical process parameters (CPPs) for nanoprecipitation method are: needle gauge, organic phase concentration, sample injection rate, should be carefully controlled to ensure robust formulation processing conditions.
Systematically experiments tested these variables (polymer concentration, surfactant type and concentration,) mixing parameters (RPM & mix time), internal and external phase volume, external phase temperature, and organic phase addition rate were considered as the CPPs. Various formulations were manufactured by changing process parameters to evaluate their impact on the final product. Each formulation was evaluated for drug loading assay (encapsulation efficiency), particle size, polydispersity index (PDI), and zeta potential (mV).
In the second phase of the study, impacted parameters from the first phase will be exhaustively studied by design of experiment using advanced statistical software. Experiments will be performed as per the formulation combination proposed by the design of the experiment and evaluated for drug loading assay (encapsulation efficiency), particle size, polydispersity index (PDI), and zeta potential (mV). The data collected will be summarized into results with using statistical software for predicting an ideal formulation profile. Predictions can later be made to develop a model for suggesting mathematically relevant formulations. These formulations will be later manufactured to validate the mathematical modeling to test various checkpoints.
During the final phase of the study, the nanocapsules will be evaluated in topical dosage forms in small scale dispersions using various pharmaceutical grade gelling agents. Topical dosage forms will be manufactured using appropriate formulation vehicles for the delivery of pharmaceutical drugs intended for localized effect. Topical dosage form will be evaluated for parameters to rapidly formulate vehicles with applications in industrial settings. IVRT, IVPT, and other physiochemical measurements will be completed to understand what major factors that are connected to the fundamental mechanisms of their behavior in-vivo.
-
Projects: Deformable Liposomes for Topical Drug Delivery
Study Participant: Zhang Julia Zhang
Liposomes are self-assembled structures formed by lipids. The use of lipid vesicles in topical delivery systems has many advantages, such as: increases in drug permeation through the stratum corneum, lowering skin irritation caused by drugs and metabolites, extending the effective time within the skin by interaction of phospholipid bilayer with the similarly structured cell membrane, and for hydrophobic drugs liposome formulations increase overall solubility without the use of additional skin irritating solvents.
However, it has been proved that classic liposomes are of little or no value as carriers for transdermal drug delivery because they do not deeply penetrate the skin, but rather remain on the upper layer of the stratum corneum. For liposomes to pass through and reach to the deeper layers of the skin, many new strategies have been developed. Among these, deformable liposomes have gained much attention in the scientific community. Deformable liposomes are prepared by combining a lipid, e.g., a phosphatidylcholine (PC) with a denaturant such as a surfactant or an alcohol.
We have conducted extensive research to develop various deformable liposomes using NSAIDs as model drugs. Liposomes were prepared by the thin film hydration method followed by sonication. These deformable liposomes were composed of phospholipid, edge activator, cholesterol and/or permeation enhancer.
These NSAID loaded liposomes demonstrated high drug entrapment rate, homogeneous particle size and improved solubility and skin permeability compared to classic liposomes.
The dermal and transdermal delivery using deformable liposomes can be a promising alternative to conventional oral delivery of NSAIDS with enhanced local and systemic onset of action and reduced side effects. Our current research goal is to incorporate these liposomal suspensions into delivery vehicles, such as hydrogels, creams or transdermal patches to eventually develop a commercially viable formulation.
-
Microneedles as Transdermal Delivery Vehicles for Neurodegenerative Disorders
Study Participants: Keyaara M. Robinson, Bozena B. Michniak-Kohn
Transdermal delivery systems (TDDS) facilitate the transport of active pharmaceutical ingredients (APIs) into systemic circulation via the skin. The transdermal route offers many advantages over traditional pharmaceutical dosage forms, including avoiding first-pass metabolism and its corresponding GI side effects, non-invasive nature, controlled delivery and ease of administration. The advantages of reducing the number of daily doses and ease of administration make transdermal patches an excellent dosage form for patients who suffer from memory loss and other cognitive disabilities.
Microneedles are small medical devices that can reduce the risk of stimulating nerve endings and can allow for the efficient delivery of many drugs through the skin for entry into systemic circulation. They are minimally invasive, are capable of provide controlled release of drugs, and are relatively simple to manufacture.
Members of the LDD developed and optimized a pressure sensitive adhesive patch formulation which was able to release efficacious levels of drug through human cadaver skin over a period of 24 hours. The objective of this research is to build upon this work to develop a microneedles-based transdermal patch for sustained release of drug at efficacious levels over a period of 3-7 days. The successful patch would allow for less frequent dosing for patients and caregivers, and possibly improve health outcomes for patients with neurodegenerative disorders.
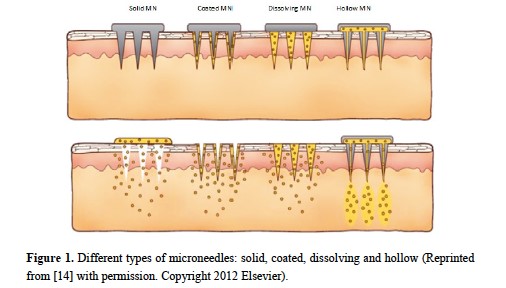
Ita, K. Pharmaceutics. 2015 Jun 29;7(3):90-105
-
Transungual (Nail) Drug Delivery

Study Participant: Vinam Puri
With foregoing successful years in topical and transdermal delivery, the Laboratory for Drug Delivery (LDD) has been conducting research in another challenging field of delivery: nail (transungual) drug delivery. Transungual drug delivery refers to the transport of drugs across the nails to achieve targeted delivery for nail diseases. Onychomycosis is the fungal disease of the nail that is growing rapidly worldwide especially in the older population. The condition involves discoloration, brittleness and thickening of the nails. It is a recurring disease seen more in toenails than in fingernails and the approaches to deliver antifungal drugs to these sites of the infection are oral and topical. The oral route poses a significant risk of severe systemic adverse effects and the topical route poses the challenge of effective permeation through the highly keratinized compact nail plate. A transungual drug transport system, if achieved, provides a better delivery route than oral treatment of fungal infections due to its better adherence, localized action and minimum systemic side effects.
Transungual drug delivery studies at the LDD have been expanded significantly with interest in deep nail layer delivery. We are working with novel formulations containing different antifungal drugs to achieve effective delivery. Starting with analytical method development and validation for quantification of antifungal drugs; and characterization and pre-treatment of human cadaver nails, we conduct drug permeation studies using vertical Franz Diffusion Cells modified with nail adapters (Figures 1 and 2).

Figure 1: Modified vertical Franz Cells assembled with nail adaptor
Figure 2: Human cadaver nails as a model for studying onychomycosis
Efforts are being made not only towards achieving drug permeation through the nail, but also understanding lateral diffusion of drugs as the nail grows. We have collaborations with our partners at TRI Princeton and other research groups with the latest technology platforms and testing capabilities like confocal microscopy, spectroscopic analysis, scanning electron microscopy, etc. to enhance our efforts in this area.
Currently, we are focusing on developing different formulation approaches with varied antifungal molecules such as terbinafine hydrochloride, econozaole nitrate, and ketoconazole, etc. to study their efficiency in achieving the required delivery. Using design of experiments and quality by design approaches, Nail patches, lacquers as well as vesicular systems such as nanoparticles, lipid nanostructures, and more loaded into carriers such as gels are being studied at the LDD. This allows investigation of effects of several factors simultaneously at a much faster rate as compared to traditional one factor at a time approach. Additionally, chemical and physical methods of permeation enhancement are being explored in conjunction with the formulation strategies. Analytical techniques employed for the characterization and quantitative analysis of these formulations employed include high performance liquid chromatography, liquid chromatography – mass spectrometry, fourier-transform infrared spectroscopy, rheology, etc.
Apart from onychomycosis, there are several other indications that can also be approached using these topical delivery approaches, such as nail psoriasis, yellow nail syndrome, paronychia, pitting of nails, and hyperkeratosis. If effective drug delivery is achieved from topical application of drug formulations, it will result in overcoming numerous challenges faced by the current treatments. The LDD aspires to make a significant contribution to these efforts by dedicated high quality research endeavors.
-
Transdermal delivery of an antiepileptic drug, using human skin and penetration enhancers in vitro
Study Participant: Amitkumar Virani
Epilepsy is a chronic neurological disorder characterized by recurrent unprovoked seizures. The abnormal discharge of neurons within the central nervous system (CNS) causes seizures. Epilepsy is one of the most common and devastating neurological disorders which is estimated to have a worldwide prevalence of about 0.5–1%. There are several antiepileptic drugs currently available to control and suppress seizures.
Antiepileptic drugs are available as tablets and suspension for oral administration. Oral administration can create difficulty for patients who cannot swallow, such as the elderly, stroke victims, patients who refuse to swallow, such as pediatric, geriatric and psychiatric patients. Transdermal drug delivery (TDDS) has gained considerable attention because this route offers several advances over other more conventional routes of drug delivery. The transdermal route of administration has been recognized as one of the potential routes for both the local and systemic delivery of drugs. Transdermal delivery for systemic activity provides several advantages, especially when drugs have low oral bioavailability due to the extensive first passage metabolism and/or a short half-life or even when drugs indicate some adverse side effects following the oral administration. On the other hand, the main limitation of transdermal delivery of drugs is that the skin layers provide high resistance to the penetrant molecules.
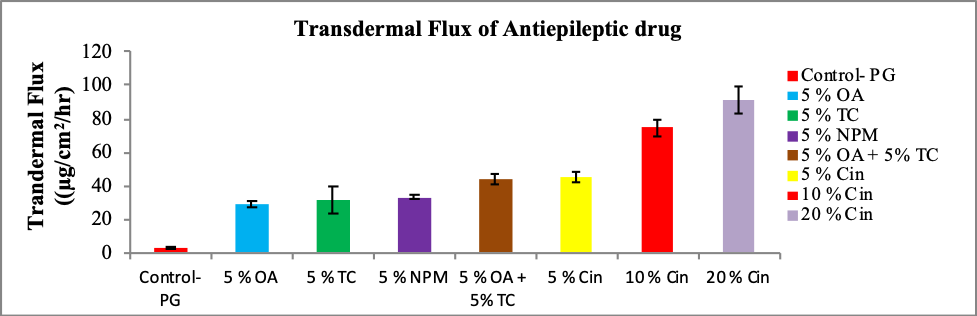
The aim of the study was to investigate the effect of various penetration enhancers on the transdermal delivery of an epileptic drug. Transcutol® P (TC), Oleic Acid (OA), Cineole (Cin) and N-methyl-Pyrrolidone (NPM) were used as penetration enhancers. Simple formulations of epileptic drug in Propylene Glycol (PG) incorporating various penetration enhancers and combination of penetration enhancers were prepared. An antiepileptic drug candidate may be any of the following: Rufinamide, Carbamezepine, Phenytoin, Clobazam and Oxcarbazepine. The ex-vivo permeation study was conducted using vertical glass Franz diffusion cells mounted with human cadaver skin. The flux and the amount permeated of an epileptic drug molecules were significantly increased with the incorporation of various penetration enhancers in the formulations containing propylene glycol as the main solvent. The long term goal of our study is to develop new epileptic transdermal formulations using this alternative route of administration for the treatment of epilepsy.