Research
Our Research group is actively involved in a range of studies from bone tissue engineering to ligament strength enhancement. Below are a few of the studies that have become staples in or research portfolio.
-

This research area includes several of the other research areas/projects.
Our lab works in conjunction with our collaborators in order to produce new biomaterials in many different forms from nanofibers to hydrogels for a variety of tissue engineering purposes.
-
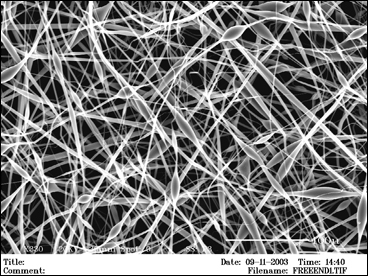
Bone is a connective tissue that is a composite by nature with an intricate hierarchical structure. Current methods for bone replacement include autografts, allografts, and metallic replacements. Our lab is developing a new tissue-engineered approach to bone replacement, repair, and regeneration using degradable, composite nano and microstructures.

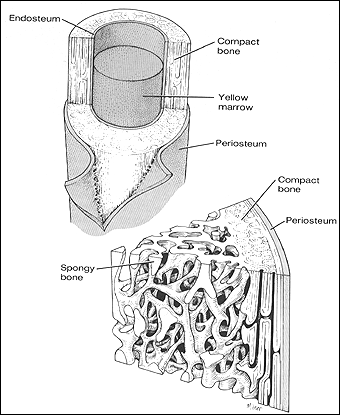
-
We are developing treatments for a number of musculoskeletal disorders by altering collagenous tissue mechanical properties. This is useful for osteoarthritis and disorders such as Ehlers-Danlos Syndrome.
Osteoarthritis (OA) is the most prevalent musculoskeletal disease in humans characterized by degeneration and loss of articular cartilage. Genetic factors, age related wear and tear processes, and acute trauma contribute to cartilage degradation and OA. Biomechanical forces increase inflammatory mediator production by chondrocytes and potentiates abnormal cartilage function in the degenerative joint.
Ehlers-Danlos syndrome is a disorder that elelcts the ability of collagen to form tight networks. This leads to weakened tissues and unstable joints.
These therapies are designed to be injectable to increase their ease of use. Our data shows their effectiveness in increasing the elastic moduli of cartilage and ligaments in vitro
-
Type I Collagen
Collagens are major structural proteins in vertebrates that store and transmit energy in the musculoskeleton. Collagens are present in most of the connective and supportive tissues in vertebrates, and they make up almost 30% of all protein in the human body and 90% of the organic material in bone. Approximately, 20 types of collagens have been identified; bones, skin, tendons, and ligaments contain mainly type I collagen; therefore, the type I collagen molecule plays an important role in energy storage in connective tissues.
The type I collagen molecule is a triple helix composed of three polypeptide chains, each exhibiting a left-handed helix. Together, these three chains form a right-handed triple helix. In collagen type I, two of these chains (termed alpha 1) have the same amino acid sequence, while the third chain (termed alpha 2) has a slightly different combination of amino acids. The triple helix is approximately 300 nm long and 1.5 nm wide with about 1000 amino acids per chain. These chains contain a glycine (Gly)-X-Y repeating pattern, where X and Y are commonly Proline (Pro) and Hydroxyproline (Hyp), respectively; however, the X and Y positions can be occupied by a number of other amino acids.
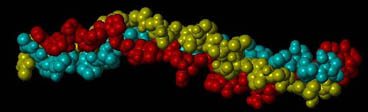
A section of a collagen molecule composed of three amino acid chains (green, red, yellow) assembled into a triple helix.
Collagens as Biomaterials
Type I collagen can self-assemble into structures called fibrils (tens to hundreds of nm in diameter) with a quarter-staggered packing array of molecules. This quarter-stagger produces a characteristic banding pattern that can be seen with electron microscopy.For the experimental aspect of this project, we are seeking to study collagen type I as a biomaterial for potential tissue engineering applications. We are employing the self-assembling nature of collagen type I to reconstitute the molecules into biomaterials such as fibers and films/membranes.
Molecular Modeling
The molecular modeling approach that we are currently using is called molecular mechanics, which is based on principles from classical physics. In this modeling approach, atoms are represented by hard spheres.
Covalent bonds are represented by spring-like connections that store potential energy whenever deviated from equilibrium (i.e., the energy stored when a spring is stretched or compressed).
Non-covalent bonds are often modeled by Lennard-Jones and Coulombic terms. The Coulombic term represents the attraction and repulsion between atoms due to their partial charges (i.e., opposite charges repel and like charges attract). The strength of this attraction/repulsion is based on the magnitude of the charges and the distance between the atoms. The Lennard-Jones term includes attractions due to van der Waals interactions and repulsions that occur when two non-bonded atoms come too close together (i.e., the hard spheres, representing the atoms, cannot overlap with each other).
With this modeling approach, the total potential energy stored in a molecule is described by a summation of various energy terms that often consider the stretching of bonds, the bending of bond angles, the twisting about bonds, out-of-plane bending around a central atom, and non-bonded interactions (Lennard-Jones and Coulombic).
We are interested in modeling and predicting the mechanical behavior of type I collagen to better understand the mechanical resiliency of this protein (i.e., how this protein stores elastic energy). This mechanical resiliency is important in the transmission of forces within collagen and in collagenous tissues such as tendons and ligaments.
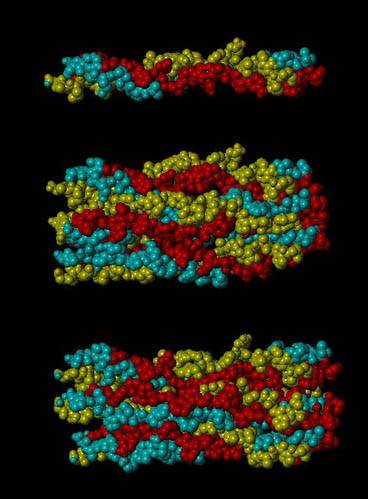 A section of the d band of type I collagen (top), the entire d band (middle), and the d band strained 5% (bottom). Each section has with 3 repeats of Gly-Pro-Hyp on the ends of each chain. This image was generated using SYBYL as part of a molecular model of type I collagen.
A section of the d band of type I collagen (top), the entire d band (middle), and the d band strained 5% (bottom). Each section has with 3 repeats of Gly-Pro-Hyp on the ends of each chain. This image was generated using SYBYL as part of a molecular model of type I collagen.
The following lists some of the resources that we have found helpful for the molecular modeling aspect of our work:
-
Muscle contraction can be interrupted by traumatic injuries to peripheral nerves (PNs) and/or skeletal muscle. After a muscle is injured, necrotic muscle fibers are removed and satellite cells are activated to help regenerate the skeletal muscle. However, this process results in scar tissue formation and loss of muscle function. Autologous muscle transplants and exogenous myogenic cells, satellite cells, and myoblasts have also been investigated with little success. Loss of skeletal muscle has no satisfactory restoration method currently. We are developing a new electrospun biodegradable, biocompatible, and conductive scaffolding for muscle tissue engineering
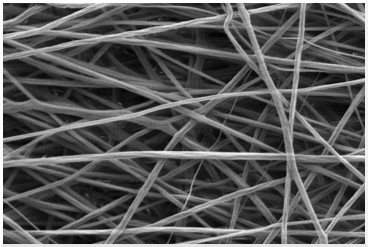
An electrospun polymer scaffold for muscle cells.
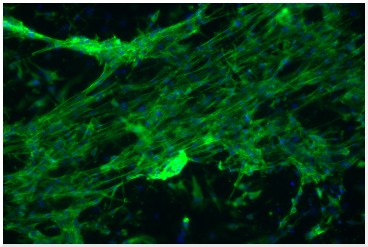 Muscle cells grown on an electrospun scaffold and stained with fluorescent dyes for actin filaments (green) and nuclei (blue).
Muscle cells grown on an electrospun scaffold and stained with fluorescent dyes for actin filaments (green) and nuclei (blue).