Daguplo, Heineken Queen: Regulation of P90 Ribosomal S6 Kinase (RSK) Phosphorylation via mTORC2
Title: Regulation of P90 Ribosomal S6 Kinase (RSK) Phosphorylation via mTORC2
Name: Heineken Queen Daguplo
Major: Biochemistry
School affiliation: Douglass Residential College, School of Environmental and Biological Sciences
Programs: Project SUPER; George H. Cook Scholars Program
Other contributors: Swati Rajput, Chadni Patel, and Estela Jacinto
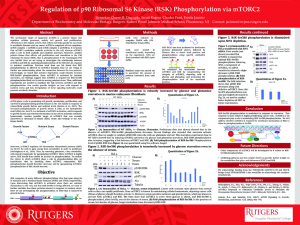
Abstract: The mechanistic target of rapamycin (mTOR) is a protein kinase that regulates cellular processes, mainly cell growth and proliferation. Alterations in the regular activity that cells in our body partake in can result in metabolic diseases such as cancer. mTOR is comprised of two complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). It is known that mTORC1 modulates anabolic activities in the cell, whereas mTORC2 plays a role in both anabolic and catabolic processes. Moreover, another protein kinase that affects cell proliferation and translation is the P90 ribosomal S6 kinase (RSK), that is phosphorylated at the homologous HM site, Ser380. Here we are trying to investigate the relationship between mTORC2 and RSK by analyzing phosphorylation of the HM sites in response to nutrient conditions. Previous work from our lab found that RSK phosphorylation is affected by mTORC2 integrity, thus we are trying to further our understanding about the connection between the two proteins. Interestingly, we found that nutrient deprivation could robustly increase RSK-Ser380 phosphorylation. Since mTORC2 is activated by nutrient starvation, our findings suggest that mTORC2 is required for RSK-Ser380 phosphorylation likely via its role in sensing nutrient stress. Thus, additional experiments are needed to elucidate the link between mTORC2 and RSK phosphorylation. Our results provide insights on how cells respond to nutrient stress and how deregulation of these signaling molecules could promote metabolic disorders.