Choudhry, Hannaan: Osmolytes and Hofmeister Salts Dynamically Regulate and Promote Mutant Huntingtin Aggregation
Title: Osmolytes and Hofmeister Salts Dynamically Regulate and Promote Mutant Huntingtin Aggregation
Name: Hannaan Choudhry
Major: Cell Biology and Neuroscience
School affiliation: Honors College, School of Arts and Sciences
Programs: Aresty – Research or Conference Funding Recipient
Other contributors: Alice Liu, Sameer Ahmad, Rawda Elsayed, Celine Molfetta, Sachin Mysore, and Maxim Yacun
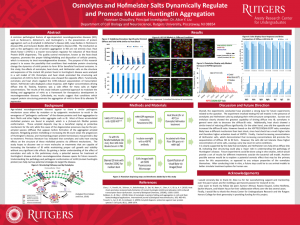
Abstract: A common pathological feature of age-dependent neurodegenerative diseases (ND), such as Parkinson’s, Alzheimer’s, and Huntington’s, is the presentation of protein aggregation such as β amyloid in Alzheimer’s disease (AD), Lewy bodies in Parkinson’s disease (PD), and Inclusion Bodies (IB) in Huntington’s disease (HD). The mechanism as well as the pathogenic role of protein aggregation in ND are not entirely clear. Heat Shock Factor 1 (HSF1) is a master transcription regulator for induction of Heat Shock Protein (HSP) chaperones. This quality control mechanism, known as the heat shock response, promotes the proper folding/refolding and disposition of cellular proteins, which is necessary to treat neurodegenerative diseases. The purpose of this research project is to assess the possibility that conditions that modulate protein structuring change the dynamics of mHtt protein to form IB for beneficial functional outcome. In this study, the effects of osmolytes, heat shock, and Hofmeister salts on the structure and expression of the mutant Htt protein found in Huntington’s disease were analyzed in a cell model of HD. Osmolytes and heat shock promoted the structuring and compaction of mHtt to form IB whereas urea showed the opposite effect. Functionally, osmolytes and heat shock negated the mHtt-Induced sequestration of transcription factors. Hofmeister salts displayed a dose response, with higher concentrations driving diffuse into IB. Toxicity, however, was a side effect for many salts at higher concentrations. The results of this study indicate a potential approach to modulate the folding and aggregation of mHtt as a framework for therapeutics development in neurodegenerative diseases. Collectively, my results suggest that conditions which support protein structuring promote the aggregation of mHtt to form IB to alleviate TF repression.