Research
Gut microbiota and immunity in infants exposed to HIV
Infants exposed to HIV but uninfected (iHEU), display heightened immune activation, inflammation, compromised growth and are at an elevated risk of neurodevelopmental delay compared to those who are HIV unexposed and uninfected(iHUU). The biological mechanisms driving these disparities are poorly understood.
Our lab investigates whether early-life gut microbiota causally contributes to these adverse outcomes in iHEU infants. We are evaluating if there is a causal relationship between early life gut microbiota in iHEU and these outcomes. We integrate clinical research using samples from mother–infant cohorts with reverse translational studies in preclinical models to uncover potential mechanistic insights linking microbial communities with developmental phenotypes.
Our goal is to define mechanisms that underlie poorer health outcomes in iHEU, and to leverage these insights to develop microbiota-targeted interventions to improve health outcomes in infants.
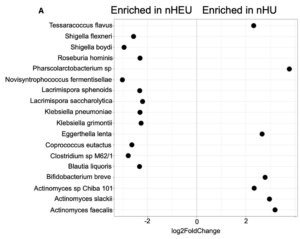
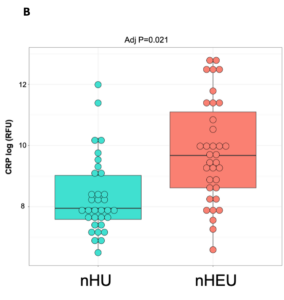
Gut microbiota and inflammatory biomarkers in neonates who are HIV-exposed but uninfected (nHEU) compared to those who are HIV-unexposed (nHU). (A) Differentially abundant gut bacteria between nHU and nHEU. (B) Plasma concentration of CRP.
Causal effect of early life gut microbiota on development in iHEU
We and others have shown that iHEU display altered gut microbiota compared to iHUU. However, whether these microbial differences cause the adverse outcomes observed in iHEU remains unknown.
To address this, we are testing the hypothesis that early life gut microbiota from iHEU impairs immune development, growth and neurodevelopment. We use gnotobiotic mouse models colonized with iHEU stool to directly assess the causal links between microbial communities and host development.
Effect of maternal antiretroviral drugs on infant microbiome, virome, and development
iHEU experience maternal HIV infection and the associated perturbed immune environment in utero. In addition, they are exposed to antiretroviral (ARV) drugs through maternal therapy during pregnancy and breastfeeding as well as their own ARV prophylaxis after birth. While combination antiretroviral therapy effectively suppresses maternal viral replication, these medication may have unintended effects that warrant closer investigation.
For example, some antiretrovirals have been shown to display antibacterial activity in vitro suggesting that antiretrovirals may directly impact the gut microbiota composition, although this remains to be robustly tested. Such drug-mediated shifts in early life microbial communities could potentially contribute to adverse outcomes reported in iHEU.
We are investigating, using animal models, how maternal combination antiretroviral therapy and early life ARV prophylaxis shape infant gut microbiome and virome, and how these changes impact immune development, growth and neurodevelopment in the offspring.