Research
-
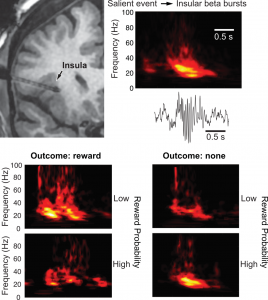
Functional imaging studies indicate that the insula encodes the salience of stimuli and deviations from expectations, signals that can mobilize cognitive resources and facilitate learning. However, there is no information about the physiological underpinnings of these phenomena beyond changing BOLD signals. To shed light on this question, we analyzed intracerebral local field potentials (LFPs) in five patients with epilepsy of both genders performing a virtual reality task that featured varying odds of monetary rewards and losses. Upon outcome disclosure, the anterior (but not the posterior) insula generated bursts of beta oscillations whose amplitudes were lower for neutral than positive and negative outcomes, consistent with a salience signal. Moreover, beta burst power was higher when outcomes deviated from expectations, whether the outcome was better or worse than expected, indicating that the insula provides an unsigned prediction error signal. Last, in relation to insular beta bursts, many higher-order cortical areas exhibited robust changes in LFP activity that ranged from spectrally nonspecific or differentiated increases in gamma power to bursts of beta activity that closely resembled the insular beta bursts themselves. Critically, the activity of these other cortical regions was more closely tied in time to insular bursts than task events, suggesting that they are associated with particularly significant cognitive phenomena. Overall, our findings suggest that the insula signals salience and prediction errors via amplitude modulations of beta bursts, which coincide with the near simultaneous recruitment of vast cortical territories.
Recent publications on this theme (asterisks indicate research trainees):
*Haufler D, Liran O, Buchanan RJ, Pare D (2022) Human anterior insula signals salience and deviations from expectations via bursts of beta oscillations. J Neurophysiol. doi: 10.1152/jn.00106.2022
-
The ability to learn that some stimuli or situations are associated with dangerous or rewarding outcomes is generally advantageous. However, such learning can also lead to a self-reinforcing cycle of harmful behaviors. Thus, it would be useful to achieve control over the network mechanisms that regulate the acquisition and expression of learned emotional behaviors. This is the objective we pursue here. Background. Principal basolateral amygdala (BLA) neurons are essential for the acquisition and expression of conditioned emotional behaviors. Yet, remarkably few of them are activated by emotionally-valenced stimuli. The solution to this paradox resides in the synchronizing influence of gamma. Indeed, gamma drastically increases firing synchrony, amplifying the impact of BLA cells on their targets. Yet, it barely alters BLA firing rates. Thus, we will study the impact of boosting or dampening BLA gamma on emotional learning. To this end, we will combine optogenetics with programmable multi-channel signal processors, known as “field programmable gate arrays” (FPGAs). Unlike computers, FPGAs allow nearly instantaneous signal analysis and conditional light stimulus delivery, providing unprecedented control over fast neuronal events like gamma, in real time. Approach. Parvalbumin (PV)-expressing interneurons play a critical role in the genesis of gamma. Thus, expression of the excitatory opsin Chronos will be restricted to PV cells, by infusing the virus AAV5-hSyn-FLEX-Chronos-GFP in the BLA of PV-cre rat. Then, to boost or dampen gamma, the optogenetic excitation of PV cells will be timed to coincide with their preferred or non-preferred gamma firing phase, respectively.
Recent publications on this theme (asterisks indicate research trainees):
*Headley DB, *Kyriazi P, Feng F, Nair S, Pare D (2021) Gamma oscillations in the basolateral amygdala: localization, microcircuitry, and behavioral correlates. J Neurosci, 41(28):6087-6101. https://doi.org/10.1523/JNEUROSCI.3159-20.2021.
*Kanta V, Pare D, *Headley D (2019) Closed-loop control of gamma oscillations in the amygdala demonstrates their role in spatial memory consolidation. Nature Communications, 10(1):3970. doi: 10.1038/s41467-019-11938-8
-
For decades, Pavlovian fear conditioning has been the dominant paradigm to study the amygdala. This paradigm fostered the view that the amygdala contains cells that signal threat and in turn generate defensive behaviors by driving downstream effectors. It has also led to the notion that the increased cue-evoked firing rates seen after conditioning are potentiated sensory responses that automatically drive conditioned behaviors (CRs). Yet, our recent work indicates that these two foundational assumptions are incorrect: activity in the basolateral (BL) nucleus is not well predicted by reward or threat contingencies but is closely linked to behavioral output. In fact, the Pavlovian paradigm is poorly suited to examine the relation between amygdala activity and behavior. The main problem is that the Pavlovian paradigm usually allows for only one CR (freezing).
As a result, it looks as if the conditioned stimulus (CS) automatically triggers the CR when in fact that’s all this paradigm allows. Moreover, because conditioning changes the likelihood that the CS will elicit the CR, it is difficult to disentangle whether training-induced alterations in activity are related to CS valence or identity, to the behaviors it elicits, or a mixture thereof. To circumvent these limitations, we examine how amygdala activity controls different conditioned behaviors using a novel task, the Risk-Reward Interaction (RRI) task, which is more ethologically relevant than the Pavlovian paradigm.
This task allows one to compare, activity related to different conditioned behaviors triggered by the same CS, in the same rats and neurons. Rats are trained to respond to the same light CS in different ways depending on where the CS is presented. In some positions, the CS signals reward availability and in others, an impending footshock. The footshock can be avoided passively or actively, depending on the rats’ position with respect to the CS. The RRI task also features conflict trials where rats must forego the reward to avoid the shock. Our preliminary results indicate that amygdala neurons exhibit multidimensional coding.
Recent publications on this theme (asterisks indicate research trainees):
*Kyriazi P, *Headley DB, Paré D (2020) Different multidimensional representations across the amygdalo-prefrontal network during an approach-avoidance task. Neuron, 107(4):717-730.e5. https://doi.org/10.1016/j.neuron.2020.05.039
*Headley DB, *Kanta V, *Kyriazi P, Paré D (2019) Embracing complexity in defensive networks. Neuron, 103(2):189-201. doi: 10.1016/j.neuron.2019.05.024
*Kyriazi P, *Headley DB, Pare D (2018) Multi-dimensional coding by basolateral amygdala neurons. Neuron, 99(6):1315-1328. doi: 10.1016/j.neuron.2018.07.036
Paré D, Quirk GJ (2017) When scientific paradigms lead to tunnel vision: lessons from the study of fear. npj Science of Learning. doi: https://doi.org/10.1038/s41539-017-0007-4
*Amir A, *Lee SC, *Headley DB, *Herzallah MM, Pare D (2015) Amygdala signaling during foraging in a hazardous environment. J Neurosci, 35:12994-3005. doi: 10.1523/JNEUROSCI.0407-15.2015
*Lee SC, *Amir A, *Headley DB, *Haufler D, Paré D (2016) Basolateral amygdala nucleus responses to appetitive conditioned stimuli correlate with variations in conditioned behavior. Nature Communications, 7:12275. doi: 10.1038/ncomms12275
-
The amygdala plays a critical role in the genesis of defensive behaviors. Moreover, it is hyperactive in humans afflicted with anxiety disorders. Thus, it is commonly believed that many anxiety disorders result, at least in part, from a dysregulation of amygdala processes normally mediating fear or defensive behaviors. Accordingly, research on the mechanisms controlling amygdala excitability might open new approaches for the treatment of anxiety disorders. This proposal aims to do just that, by studying the influence of midline thalamic (MTh) nuclei on the amygdala. Prior studies on thalamic influences over the amygdala have focused on inputs arising from the posterior thalamus, particularly from the medial portion of the medial geniculate nucleus. Yet, a number of tracing studies have revealed that MTh nuclei also contribute massive projections to the basolateral (BLA) and central (CeA) amygdala. However, other than anatomical data, little is known about the role of these strong glutamatergic inputs. The work proposed here aims to shed light on the influence of MTh inputs to the amygdala.
Recent publications on this theme (asterisks indicate research trainees):
*Ahmed N, Pare D (2023) The basolateral amygdala sends a mixed (GABAergic and glutamatergic) projection to the mediodorsal thalamic nucleus. J Neurosci. doi: 10.1523/JNEUROSCI.1924-22.2022
*Herzallah MM, *Amir A, Paré D (2022) Influence of rat central thalamic neurons on foraging behavior in a hazardous environment. J Neurosci, . doi: 10.1523/JNEUROSCI.0461-22.2022
*Ahmed N, *Headley DB, Paré D (2021) Optogenetic study of central medial and paraventricular thalamic projections to the basolateral amygdala. J Neurophysiol. doi: 10.1152/jn.00253.2021
*Amir A, Paré JF, Smith Y, Paré D (2019) Midline thalamic inputs to the amygdala: Ultrastructure and synaptic targets. J Comp Neurol, 527(5):942-956. doi: 10.1002/cne.24557
-
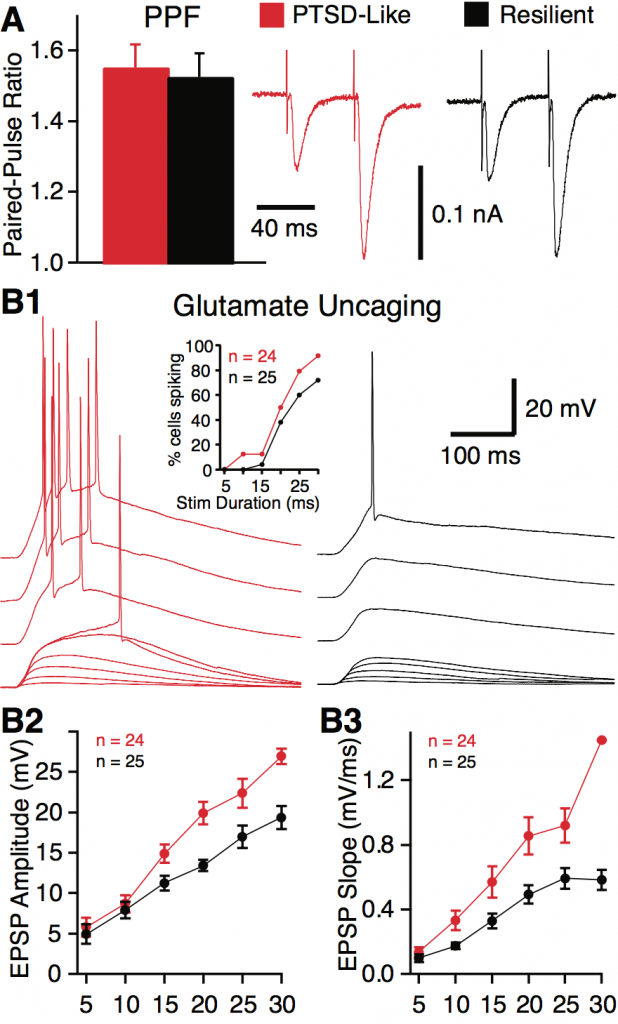
It is commonly believed that BNST generates long lasting anxiety-like states in response to diffuse contingencies but that it is not involved in the expression of learned fear responses to discrete sensory cues, the latter depending on the amygdala. In contrast, our previous work indicates that BNST activity contributes to cued fear in two ways: by prolonging fear responses long after the threatening stimulus has ended (hereafter termed temporal generalization of fear) and by allowing different (safe) cues to also trigger fear (hereafter termed stimulus generalization of fear). Since experiencing fright long after the threat has passed or in response to safe stimuli are hallmarks of anxiety disorders, understanding how BNST contributes to fear generalization is an issue of considerable translational significance. We are currently testing the hypothesis that through its connections with the amygdala and downstream effector regions, BNST contributes to the generalization of learned fear responses to cues.
Recent publications on this theme (asterisks indicate research trainees):
*Gungor NZ, Pare D (2016) Functional heterogeneity in the bed nucleus of the stria terminalis. J Neurosci, 36:8038-49.
*Rodriguez-Sierra, *Goswami S, *Turesson HK, Pare D (2016) Altered responsiveness of BNST and amygdala neurons in trauma-induced anxiety. Translational Psychiatry, 6:857.
*Gungor NZ, *Yamamoto R, Pare D (2015) Optogenetic study of the projections from the bed nucleus of the stria terminalis to the central amygdala. J Neurophysiol, 114:2903-2911.
*Gungor NZ, Pare D (2014) CGRP inhibits neurons of the bed nucleus of the stria terminalis: implications for the regulation of fear and anxiety. J Neurosci, 34:60-65.
*Haufler D, *Nagy FZ, Pare D (2013) Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learning & Memory, 20:633-41
-
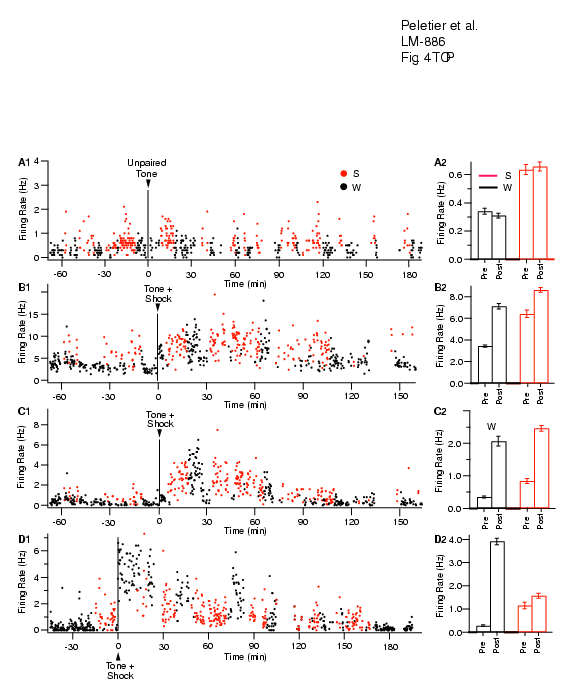
Emotionally charged events are generally better remembered than neutral ones. The available data suggests that the amygdala is responsible for this modulation of memory consolidation by emotions. In short, neuromodulators released in emotionally arousing conditions would alter the activity of basolateral amygdala (BLA) neurons in the hours after the learning episode.In turn, these changes would facilitate synaptic plasticity elsewhere in the brain. This view emerged from behavioral analyses of the effects of intra-amygdaloid drug injections; however, we have no direct evidence that these events take place. Moreover, we do not know how transient increases in neuromodulator levels could exert a sustained effect on BLA neurons. Considering the devastating psychological consequences of extreme stress, understanding how the amygdala modulates memory emerges as an issue of fundamental importance. Thus, we are investigating this issue using a combination of behavioral experiments, multisite in vivo extracellular recordings and in vitro whole cell (standard and perforated patch) recordings in amygdala slices.
Recent publications on this theme (asterisks indicate research trainees):
*Paz R, Paré D (2013) Emotional modulation of memory circuits by the amygdala. Curr Opin Neurobiol, 23:381-6.
*Headley DB, Paré D (2013) In sync: gamma oscillations and emotional memory. Front Behav Neurosci, 7:170.
*Popa D, *Duvarci S, *Popescu AT, Lena C, Paré D (2010) Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci USA, 107:6516-6519.
*Popescu A, *Popa D, Paré D (2009) Coherent gamma oscillations couple the amygdala and striatum during learning. Nature Neuroscience, 12:801-807.
*Popescu AT, *Saghyan AA, Paré D (2007) NMDA-dependent facilitation of corticostriatal plasticity by the amygdala.Proc Natl Acad Sci, 104:341-346.
*Duvarci S, Pare D (2007) Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci, 27:4482-4491
-
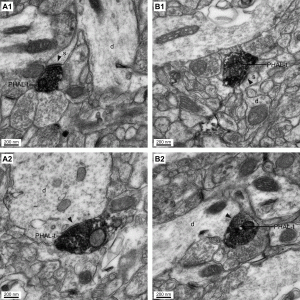
The rhinal cortices play a critical role in high-order perceptual/mnemonic functions and constitute the main route for impulse traffic to and from the hippocampus. However, there is a discrepancy between anatomical and physiological data about this network. Tracing studies indicate that the perirhinal cortex forms strong reciprocal connections with the neocortex and entorhinal cortex. In contrast, physiological studies indicate that perirhinal transmission of neocortical and entorhinal inputs occurs with an extremely low probability. Currently, our work aims (1) to shed light on the inhibitory mechanisms that limit impulse traffic through the rhinal cortices, (2) to identify the afferents that allow the rhinal cortices to overcome this inhibition, focusing on inputs from the medial prefrontal cortex and amygdala, and (3) to reveal the network properties of the rhinal cortices that overcome this inhibition in order to gate and assocaite neocortical inputs.
Recent publications on this theme (asterisks indicate research trainees):
Samarth P, Ball JM, *Unal G, Pare D, Nair SS (2016) Mechanisms of memory storage in a model perirhinal network. Brain Struct Funct, 222:183-200.
*Unal G, *Apergis-Schoute J, Paré D (2011) Associative properties of the perirhinal network. Cereb Cortex, 22:1318-1332.
*Apergis-Schoute J, *Pinto A, Paré D (2007) Muscarinic control of long-range GABAergic inhibition within the rhinal cortices. J Neurosci, 27:4061-4071.
*Paz R, *Bauer EP, Paré D (2007) Learning-related facilitation of rhinal interactions by medial prefrontal inputs. J Neurosci, 27:9369-79.
*Paz R, *Pelletier JG, *Bauer EP, Paré D (2006) Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nature Neuroscience,9: 1321-1329
-
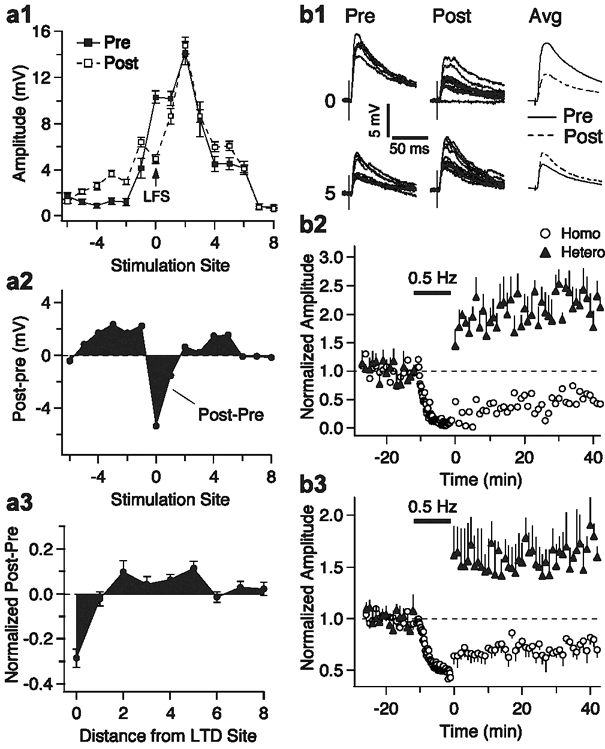
This theme is closely related to (1) our interest in understanding the modulation of memory consolidation by the amygdala, and (2) the ability of the amygdala to form a trace of some stimulus contingencies. With respect to the first issue, our work aims to identify how amygdala axons promote synaptic plasticity in target structures. Our working hypothesis is that there is a higher NMDA to AMPA ration at amygdala synapses and that this property would promote heterosynaptic plasticity of other inputs to the same cells. With respect to the second theme, we are currently examining the ability of inputs to various amygdala cell types to undergo activity-dependent plasticity.
Recent publications on this theme (asterisks indicate research trainees):
Kim D, Pare D, Nair SS (2013) Assignment of model amygdala neurons to the fear memory trace depends on competitive synaptic interactions. J Neurosci, 33:14354-8.
*Popescu AT, *Saghyan AA, *Nagy FZ, Paré D (2010) Facilitation of corticostriatal plasticity by the amygdala requires Ca2+-induced Ca2+ release in the ventral striatum. J Neurophysiol, 104:1673-1680.
*Samson R, Paré D (2005) Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J. Neurosci., 25: 1847-1855.
*Royer S, Paré D (2003) Conservation of total synaptic weights via inverse homo- vs. heterosynaptic LTD and LTP. Nature 422: 518-522
-
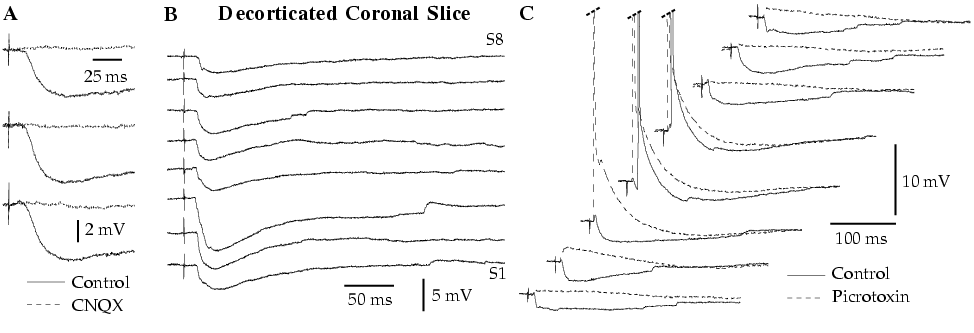
The amygdala is a nucleated structure of the temporal lobe critical for the expression and learning of fear responses. As a result, it is widely believed that disturbances in amygdala physiology underlie human anxiety disorders. However, the inner workings of the amygdala remain obscure, in part because we do not understand its intrinsic network. This is why we are studying the intrinsic circuit of the amygdala, using in vitro and in vivo electrophysiological methods as well as single-cell labeling and immunohistochemistry at the light and electron microscopic level. As a first step, we focus on the lateral amygdaloid nucleus (LA) because it is the main input station of the amygdala for sensory afferents.
Recent publications on this theme (asterisks indicate research trainees):
Paré D, *Duvarci S (2012) Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol, 22:717-723.
*Amir A, *Amano T, Paré D (2011) Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol, 105:3054-3066.
*Popescu AT, Paré D (2011) Synaptic interactions underlying synchronized inhibition in the basal amygdala: evidence for existence of two types of projection cells. J Neurophysiol, 105:687-696.
*Samson R, Paré D (2006) A spatially structured network of inhibitory and excitatory connections directs impulse traffic within the lateral amygdala. Neuroscience 141(3):1599-609.