Research
Introduction
Fear is defined as an evolutionary adaption and emotion that triggers a series of defense mechanisms in response to threatening events. While animals possess forms of innate fear, learned fear is formed by associating a neutral stimulus with a threatening event. This can be assessed through the Pavlovian fear-conditioning paradigm. In fear conditioning, a conditioned, neutral stimulus (CS), such as a room, box, tone, etc., becomes associated with an aversive, unconditioned stimulus (US), such as a shock. Through the paradigm, the neutral stimulus will eventually be enough to elicit a fearful response, such as freezing. This forms a learned fear. The neural circuitry responsible for learned fear is associated with the amygdala, hippocampus and several other brain areas. The amygdala is an essential component in the brain for learning fear and identification of amygdala enriched genes is critical for understanding how learned fear is processed (Shumyatsky et al., 2002).
The Role of GRP and GRPR in Learned Fear
|
|
|
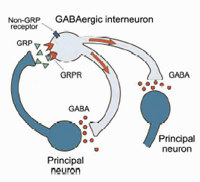
In collaboration with the laboratory of Catherine Dulac (Harvard University), we used the differential screening of single-cell cDNA libraries derived from individual principal neurons from the basal lateral complex of the amygdala (BLA) to identify severed amygdala-enriched genes. We found that the GRP gene, which encodes gastrin-releasing peptide, is expressed in the lateral nucleus of the amygdala (LA) as well as in the efferent areas that provide auditory CS inputs to the BLA during fear conditioning. We also found the GRP receptor (GRPR) is expressed in inhibitory GABAergic interneurons, which regulate signals that converge onto the lateral nucleus during fear conditioning. Collaboration with the laboratory of Vadim Bolshakov (Harvard Medical School) concluded mice that lack the GRPR have increased long term potentiation (LTP). This leads to more persistent long term memory in both cued and contextual fear conditioning. Specifically, GRPR determine how stimulated inputs to principle neurons are processed during fear conditioning through feedback inhibition. Thus, GRP and GRPR are essential for inhibitory control of the neural circuitry of fear (Shumyatsky et al., 2002).
The Role of Stathmin in Learned Fear
We also identified stathmin as an amygdala–enriched gene that is expressed in pyramidal neurons in BLA and efferent projectors to BLA, sending both US and CS information to it. Stathmin knockout mice showed decreased LTP and performed worse during fear conditioning, In addition, stathmin knockout mice also showed deficit in innate fear. Therefore, stathmin is essential for regulating the neural mechanisms behind both learned fear and innate fear. Because stathmin is a gene that regulates the microtubule network in the brain, we believe that microtubule dynamics is a major component of memory and various behaviors. (Shumyatsky et al., 2005).
The Role of Stathmin in Parental and Social Behaviors in Mice
Because knockout mice for stathmin showed inability to assess danger, this expands to female mice losing motivation in retrieving pups and the ability to locate safer environments. While other maternal behaviors were still present, such as sniffing, nesting, and pup approach, stathmin-deficient females could not efficiently retrieve their pups from a harmful environment. This indicates that microtubule networking does not only affect learning, but also maternal protection over pups in the presence of danger. (Martel et al., 2008). Surprisingly, their social behavior was enhanced.
How Microtubules Affect Learning and Memory in Mice
Learning causes changes in microtubule stability that are regulated by stathmin. In the first phase of changes in stathmin and microtubules, stathmin becomes dephosphorylated, promoting stathmin-tubulin binding and destabilization of the microtubules in the dentate gyrus. In the second phase, stathmin becomes hyperphosphorylated, causing microtubule stability to increase and the GluA2 subunit of AMPA receptors to be transported to the synapse. Changing stathmin levels and blocking GluA2 endocytosis changes the regulation of microtubular stability, thus changing the mechanisms behind learning and memory in mice (Uchida et al., 2014). We have shown that similar changes accompany aging in mice pups.