Research
Cancer cell metabolism
We study how nitrogen metabolism affects liver cancer development. This project is supported by 2 NIH R01 grants: one is to focus on the role of glutamine synthetase, and the other is to focus on the urea cycle enzymes. These two ammonia detoxification pathways play important roles in normal liver function. Defective ammonia clearance leads to pathological disorders such as encephalopathy. Recent literature and our own findings indicate that both glutamine synthesis and urea cycle pathways are involved in liver cancer development. Clinical data also indicate a strong correlation between defective ammonia clearance and liver cancer. We are testing the hypothesis that defective ammonia clearance is a risk factor and plays a major role in liver cancer development. To do this, we will modulate the expression of several ammonia-clearance enzymes and test the effect in mouse liver cancer models. We also study how oncogenes such as beta-catenin regulate the expression of these enzymes including glutamine synthetase and urea cycle enzymes.
Han X, Shen J, Yan J, Tacke R, Dai W, Mao Q, Daguplo HQ, Liu S, Islam A, Liu T, Koch MC, Lin RZ, Li H, Anthony T, Xie P, Zhang L, Gao S, Simon MC, Chen X, Yang J, Su X, and Zong WX. Impaired nitrogenous waste clearance promotes hepatocellular carcinoma. Sci Adv. 2026. Jan. 9. PMID: 41512056. DOI: 10.1126/sciadv.aec0766.
Dai W, Shen J, Yan J, Bott AJ, Maimouni S, Daguplo HQ, Wang Y, Khayati K, Guo JY, Zhang L, Wang Y, Gao S, Valvezan A, Ding WX, Chen X, Su X, and Zong WX. Glutamine synthetase limits b-catenin-mutated liver cancer growth by maintaining nitrogen homeostasis and suppressing mTORC1. J Clin Invest. 2022 Oct 18:e161408. PMID: 36256480. DOI: 10.1172/JCI161408.
Bott AJ, Shen J, Tonelli C, Zhan L, Sivaram N, Jiang YP, Yu X, Bhatt V, Chiles E, Zhong H, Maimouni S, Dai W, Velasquez S, Pan JA, Muthalagu N, Morton J, Anthony TG, Feng H, Lamers WH, Murphy D, Guo JY, Jin J, Crawford HC, Zhang L, White E, Lin RZ, Su X, Tuveson D, and Zong WX. Glutamine anabolism plays a critical role in pancreatic cancer by coupling carbon and nitrogen metabolism. Cell Rep 29:1287-1298. (2019). PMID: 31665640. DOI: 10.1016/j.celrep.2019.09.056.
Bott AJ, Peng IC (co-first author), Fan Y, Faubert B, Zhao L, Li J, Neidler S, Sun Y, Jaber N, Krokowski D, Lu W, Pan JA, Powers S, Rabinowitz J, Hatzoglou M, Murphy DJ, Jones R, Wu S, Girnun G, and Zong WX. Oncogenic Myc induces expression of glutamine synthetase through promoter demethylation. Cell Metab 22:1068-1077. (2015). PMID: 26603296. DOI: 10.1016/j.cmet.2015.09.025.
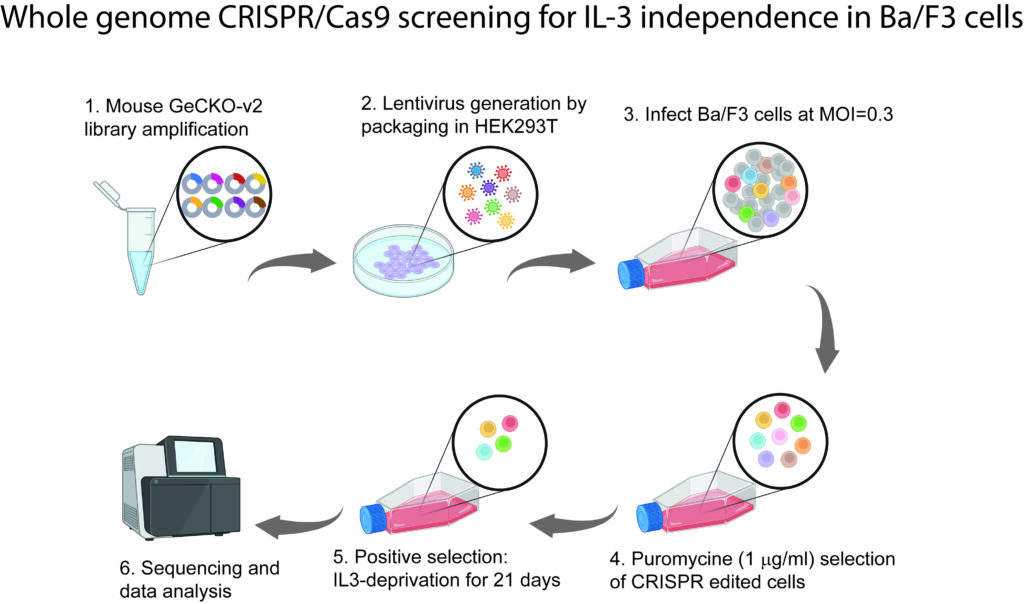
Novel tumor suppressors in B-cell lymphoma
We are developing a new research area to study the roles of several novel tumor suppressor genes that we recently identified in B-cell lymphoma. Via a CRISPR/Cas9 screening in an interleukin 3 (IL3)-dependent pro-B cell line Ba/F3, we have identified a number of genes whose loss of function leads to IL3 independence. We are currently studying three genes that are localized to human chromosome 6q, namely OSTM1, ZBTB24, and SLC35A1. These genes have different molecular functions: OSTM1 is a putative ubiquitin E3 ligase, ZBTB24 is a transcriptional factor, and SLC35A1 is a sialic acid transporter that facilitates protein sialylation. Our preliminary data indicate that silencing these genes leads to increased cell growth and transformation, and combined genetic ablation with a known tumor suppressor CDKN2A in B-cell lineage leads to enhanced B-cell lymphoma in mouse models. We are characterizing these mouse models and studying the molecular mechanisms how they regulate cell growth, survival, and transformation. Ultimately, we aim to uncover the molecular mechanisms as to how these novel tumor suppressors function, and to develop new therapeutic strategies by targeting these pathways in B-cell malignancies.