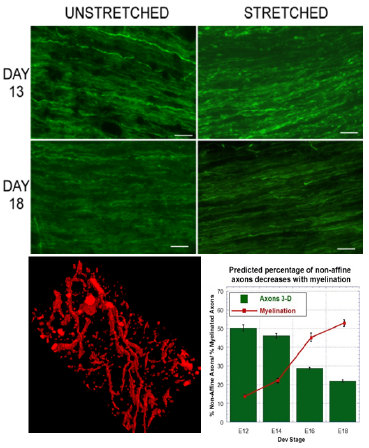
Axonal injury from stretch is one of the causes of functional damage in the central nervous system. There is a correlation between tissue-level stretch and the degree of morphological and functional damage observed. Previous studies have observed that axonal injury occurs non-uniformly, implying that axon microstructure influences the injury profile. Microstructure includes features such as axon geometry, axon tortuosity, degree of myelination, and linkage to the surrounding matrix. By extension, these features influence how axons kinematically behave due to stretch.
Kinematic models have been utilized to help explain the discrepancy and wide variability in measured in vivo injury thresholds. Our group employs a ‘coupling’ model to assess axon kinematics, where axon tortuosity is used as a metric to classify the type of behavior. In this model, axons are surmised to exhibit one of three types of kinematic behavior: non-affine, affine, and switching. In non-affine modes, axons are uncoupled to the matrix, and stretch independently of one another. At the other extreme, ‘affine’ behavior occurs when axons are coupled completely to the matrix, and stretch together, conferring more composite-like behavior. In most cases though, axons exhibit an intermediate ‘switching’ behavior, where they initially show non-affine kinematics, but switch to affine as stretch increases, and axon tortuosity decreases. Our group has characterized these features in 2-D, and is now working to extend this kinematic model to 3-D to better predict injury thresholds. Additionally we are currently probing the role of axo-glial proteins, caspr and neurofascin, in generating these coupling phenomena.