We developed an in vitro microfluidic system with two inlets which we can inject combinations of modified or unmodified type-I collagen hydrogels. These hydrogels may contain gradients of covalently grafted bioactive peptides, including laminin fragments such as IKVAV and YIGSR, or of mechanical stiffness using a natural crosslinker such as genipin. In vitro results using dorsal root ganglia explants from E8 chick embryos have demonstrated that neurite outgrowth is biased down a gradient of stiffness compared to its converse. Neurites also grow significantly longer up steep gradients of YIGSR, shallow gradients of IKVAV, and in combination compared to unmodified collagen controls.
Tissue Engineering
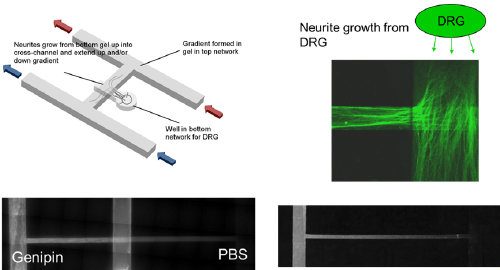
Photo-active, Thermoreversible Type-I Collagen
Type-I collagen hydrogels are often used for soft tissue engineering applications as they self-assemble into a fibrillar gel in vivo and are biocompatible, biodegradable, and naturally support cell adhesion and growth. While these hydrogels are easily amenable to chemical modifications, they have been criticized for their weak mechanical properties and lack of spatial control of bioactive/mechanical patterns. Our lab covalently grafted methacrylic acid to the free amines on the lysine residues of type-I collagen to synthesize collagen methacrylamide (CMA). CMA can be photocrosslinked in the presence of long-wave UV light and a photoinitiator for specific spatiotemporal control of chemical and mechanical patterns.
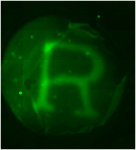
Additionally, CMA can thermo-reversibly self-assemble, forming a liquid suspension at cool temperatures, and re-assembling into a hydrogel at physiological temperature. Currently, we are using CMA for tissue engineering strategies, such as the design of specific 3-D cell microenvironments and methods for free form fabrication for controlled tissue architectures. For example, below are pictured two photo-masks next to opaque CMA scaffolds generated by exposing CMA gels to UV light through the masks, and then “cold-melting” the unreacted CMA away.