Mitochondrial functions during cancer evolution
Mitochondria are multifaceted subcellular organelles actively participating not only in energy production, but also in signal transduction, metabolism, and cell fate (Nat. Rev. Mol. Cell. Biol, 2020). All these processes are dysregulated in diseases, including cancer.
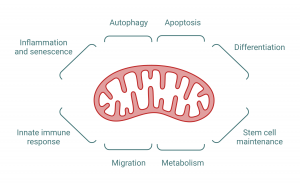
How do mitochondria adapt to facilitate tumor growth? How do these organelles orchestrate their functions within the cell throughout cancer evolution? How do mitochondria deal with stress during disease progression or after therapy? We aim to address these questions by applying a combination of state-of-art microscopy and biochemical approaches. Our goal is to advance our understanding of the mechanistic interplay between mitochondrial homeostasis and cellular fitness in malignant cells, ultimately leveraging this knowledge to translational applications and benefiting human health.
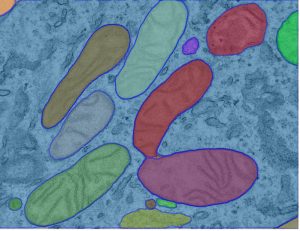
Mitochondrial dynamics in drug-resistant leukemia
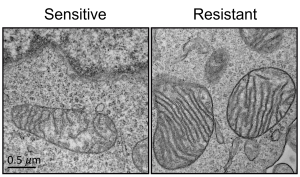
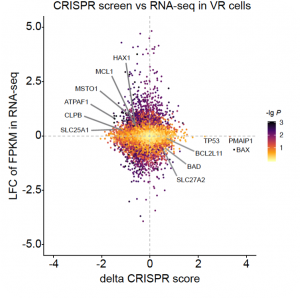
Acute myeloid leukemia (AML) is the most common leukemia in adults and it is one of the most difficult-to-treat blood cancers. Although novel targeted therapies have been recently introduced into the clinics for the treatment of AML, unfortunately many patients do not respond to the treatments or in many cases AML comes back. Recently, using electron microscopy, CRISPR technologies and biochemistry, we discovered that mitochondria in AML cells undergo morphological changes resulting in drug resistance (Cancer Discovery, 2019).
We hypothesize that mitochondrial structural adaptations may drive tumor evolution upon targeted treatment, and may be a predictor of treatment response.
The primary focus of our laboratory is to delineate the role of mitochondrial dynamics in drug resistance in AML and to address the clinical potential of targeting this cellular feature in acute leukemias. To achieve these goals, we will take advantage of cutting-edge high-resolution microscopy, biochemistry approaches, novel mouse models, and new lead compounds.