SRDR+ 5.2. Setting up Arm or Diagnostic Test Suggestions
There are two tabs within SRDR+ that have special ways for setting up questions: Arms (or Diagnostic Tests) and Outcomes (or Diagnoses). These tabs have different names depending on whether you are carrying out a diagnostic accuracy project (in which case we are talking about Diagnostic Tests and Diagnoses) or any other type of project (in which case we are talking about Arms and Outcomes).
Here, we’ll show you how to set up arms and provide some tips on useful ways to do this.
- 5.2.1 Difference Between Arms and Diagnostic Tests
- 5.2.2 How to Name Arms
- 5.2.3 When Things Get Much More Complicated: Clinical Practice Guidelines
- 5.2.4 How to Set Up and Name Diagnostic Tests
5.2.1 Difference Between Arms and Diagnostic Tests
First, here is the Arm setup page for projects that are not answering Diagnostic Accuracy questions.
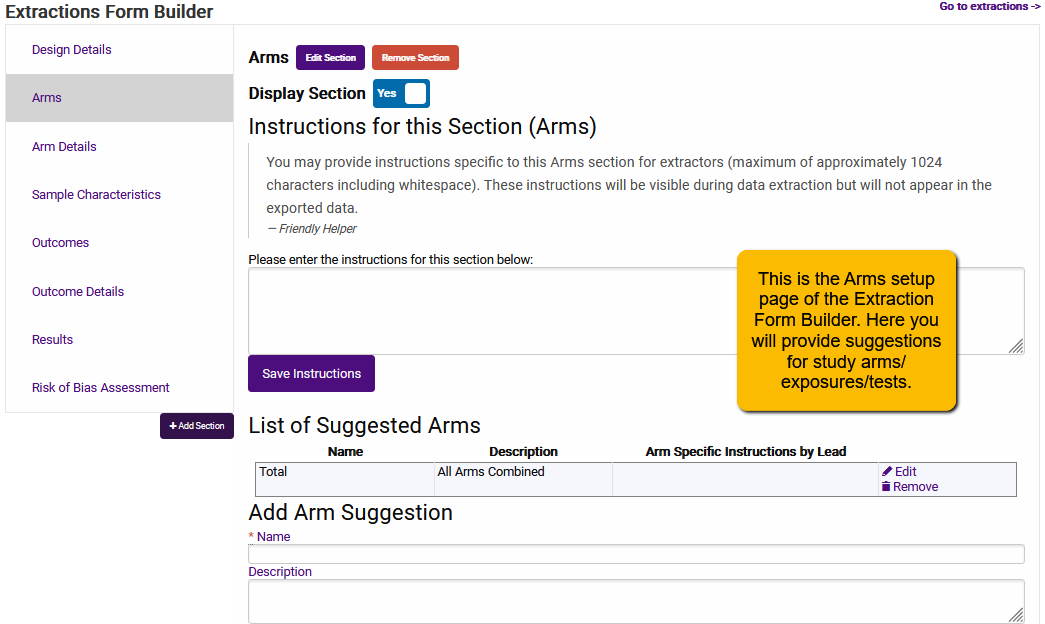
Here is the page for projects set up to answer Diagnostic Accuracy questions. You’ll notice that a couple of tabs are different.
You’ll notice that what you are doing with Arms (or Diagnostic Tests) when you are setting up the template is providing the extractor with suggestions. Think of it as building out a menu that extractors can quickly and easily draw from when they are specifying the arms in a research study.
The only field you need to enter here is the Name field (best not to enter any description as the descriptions are best left to the extractor and the peculiarities of the particular article).
5.2.2 How to Name Arms
One thing to realize up front is that authors of different articles may name what are essentially the same arms differently. For instance, let’s say our PICO question is: Among people with mental illness, what is the effectiveness of a multi-component weight loss program compared to usual care for managing weight status? We have two studies that name what are essentially the same arms different ways:
- Smith 2018: Treatment arm name, “Lifestyle Intervention”; Comparison arm name, “Usual care”
- Jones 2019: Treatment arm name, “Active Life Intervention”; Comparison arm name, “Standard care”
The obvious problem here is that if the extractors name their arms exactly as the authors did, then when you go to carry out the analysis you will have to sort through all the different names to figure out which are your intervention of interest (the “I” in your PICO) and which are your comparator arms (the “C” in your PICO). If extractors are not provided structure and training up front, this can mean a huge amount of work cleaning up the arm names in the analysis phase of your project.
So, in the simplest situation where there is just one treatment (or exposure or test) condition and one comparator condition, then we can (1) use very simple and generic arm suggestions, and (2) train the extractors how to identify which is which.
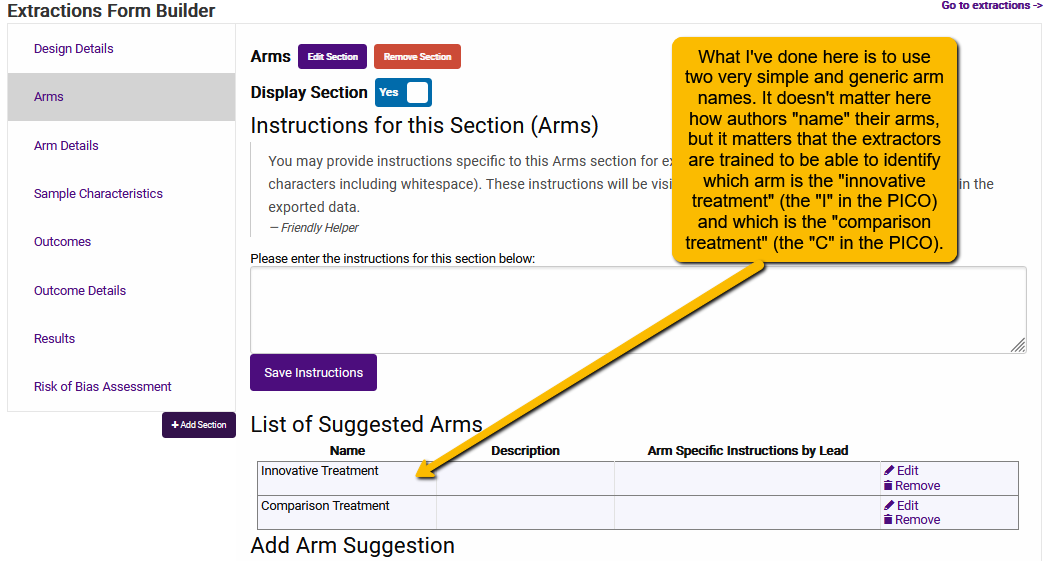
If you are interested in a very specific and commonly labeled surgery, treatment, drug, etc., or, if the comparator condition is always placebo, then you can provide a suggest more descriptive than “innovative treatment” or “comparison treatment”. In this case, setting up arms is simple.
But things aren’t always this simple. What if you know that the article you are interested in sometimes have two intervention arms (e.g., nutrition versus nutrition + exercise)? Well, in this case you can plan ahead and provide a suggestion for a third intervention arm (again, either going completely generic or more descriptive depending on your project).
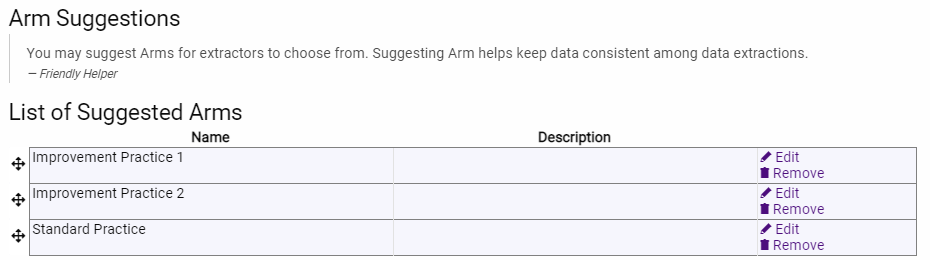
Now, a word of caution: if you are using multiple generic labels for the innovative or (as above) “improvement” practice or intervention, and you are double extracting data from articles (i.e., two data extractors working separately on each article), then this can cause confusion at the Consolidation phase of the project (that is, where a third person reconciles differences between extractors). Extractor 1 might label an arm “Innovative Practice 1” while the other extractor labels the same arm “Improvement Practice 2”. In this case, recommend to the extractors that they use a short description to differentiate the two innovation arms (or, less frequently, two comparison arms).
You may also want to have extractors communicate with each other ahead of time which arm will be labeled Practice 1 or Practice 2. If this is not feasible, then you may want to come up with a more descriptive (rather than generic) label for multi-arm studies. Again, training extractors ahead of time is key!
But you may be wondering how to capture detail about the arms if you assign only generic labels. We’ll cover that in the next section.
5.2.3 When Things Get Much More Complicated: Clinical Practice Guidelines
For single question systematic reviews, the above approach is generally fine. But, how about projects that encompass many questions (like creating Clinical Practice Guidelines)? In this case, you may need several arm suggestions. Always try to plan for how to make it easier on your data extractors (less frustration, less time, fewer mistakes). For example:
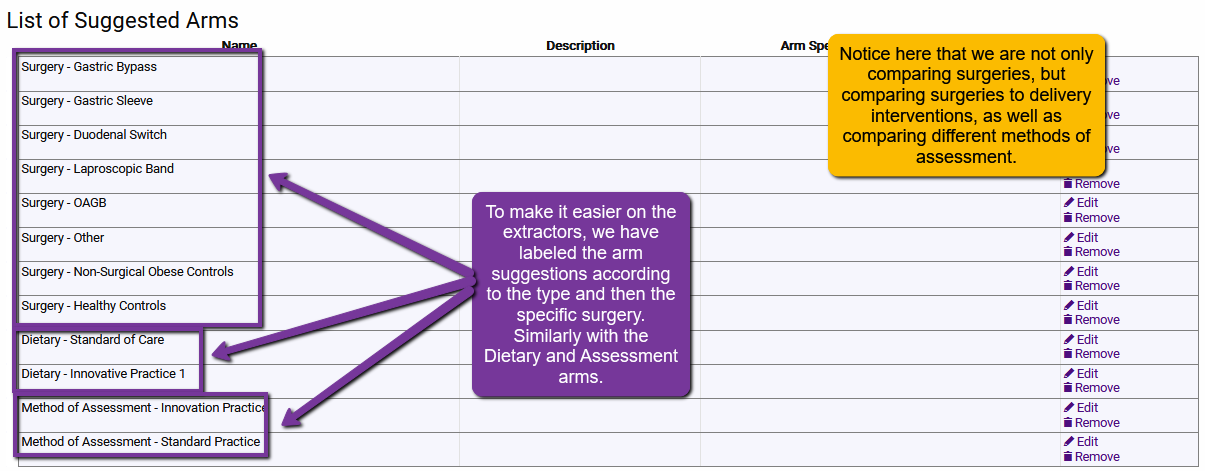
Why not just have every question for a clinical practice guideline be its own separate project within SRDR+? That is certainly an option and may be best for some projects. However, when article may apply to several clinical practice questions, it may be more efficient to keep them within the same project to avoid unnecessary duplicate (or triplicate!) extractions.
Remember, you can use multiple key questions and can assign different questions (data entry fields) to these key questions. It takes a little planning and organization but may actually help you in the end.
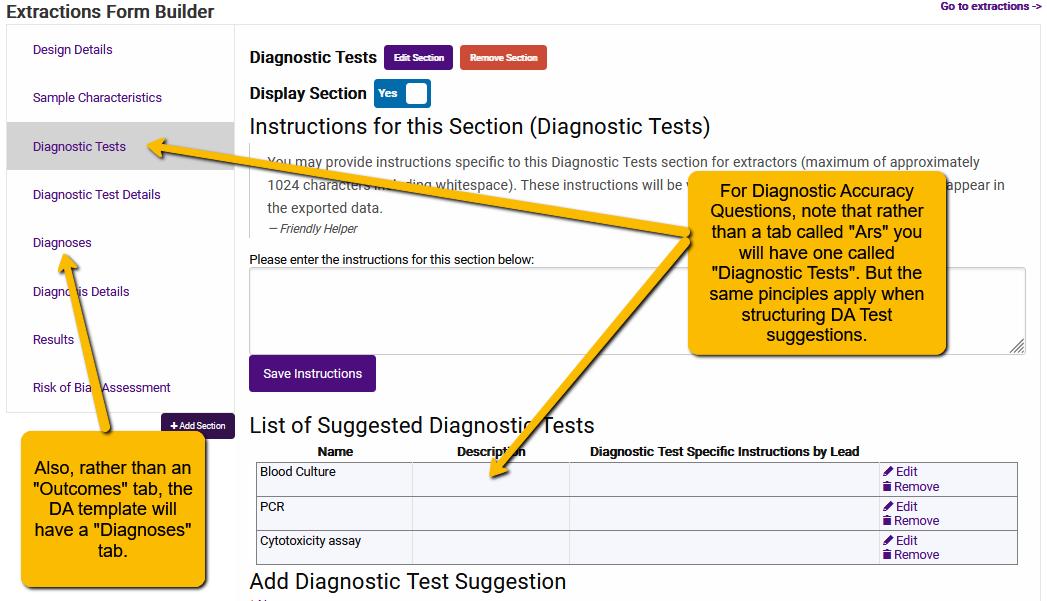
5.2.4 How to Set Up and Name Diagnostic Tests
If you are doing a Diagnostic Accuracy systematic review, there is a little prep work you need before you can set up the Diagnostic Tests (and Diagnoses).
You first have to tell SRDR+ that your Results are going to be in the proper format (which is different than other types of systematic reviews). So, when you go to the Results tab for the first time, you will see the following:
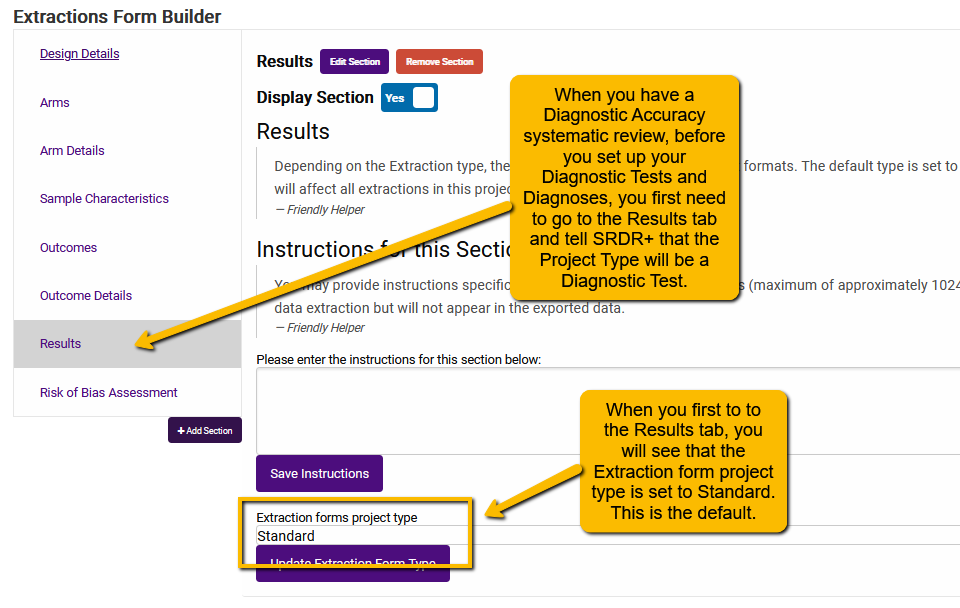
Simply use the dropdown for the Extraction forms project type field and select Diagnostic Test and click the Update Extraction Form Type. You will then see the following:
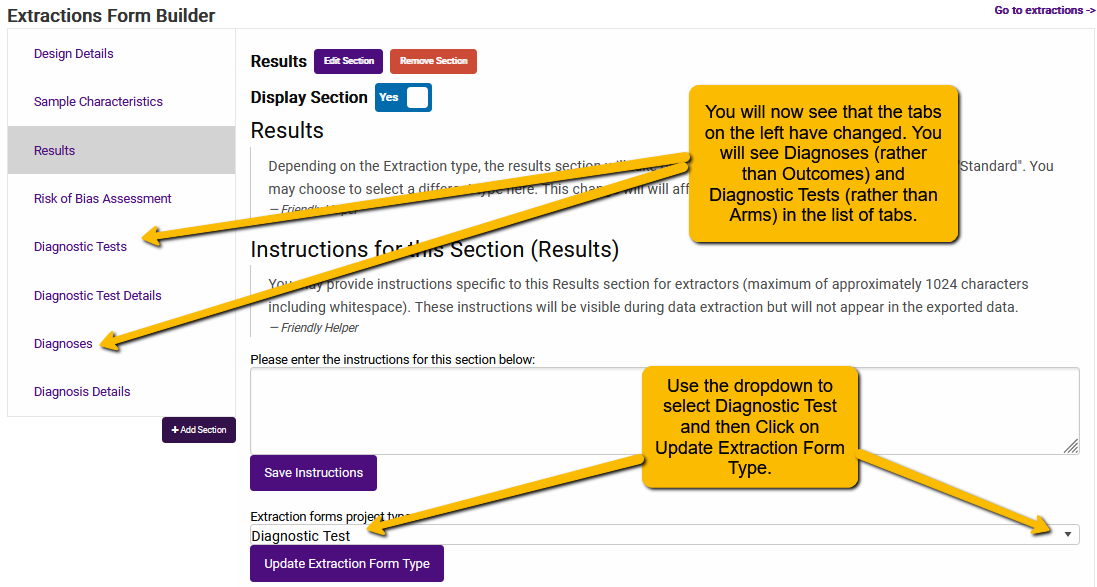
When naming the Diagnostic Tests, the same basic principles apply that we mentioned when naming Arms: keep it simple.
For instance, if you are familiar with polymerase chain reaction (PCR) tests, you will know that there are different types. Rather than have extractors enter lots of detail about the tests (or, heaven forbid, give what is essentially the same test different names) in the Diagnostic Tests page, you should keep the test suggestions simple (e.g., tests of a common type, like PCR or blood culture) and capture detail on the tests in the Diagnostic Test Details page.