Indigo is a hot topic right now, hot in the dyeing community, and hot among scholars who work on dyes. I was recently at a conference on colors where half the papers were on indigo. I was at another on craft, where the only two papers on colors were also on that same blue dyestuff (one of those two was mine, since the green on Sterne’s page is half indigo). That seems odd. But I think it is because indigo uniquely combines two different qualities that make it such a popular dye. In the first place, it is exceptionally common in the natural world. Over two hundred plants yield enough indigo precursors that they could be plausibly cultivated for that dyestuff. And, in the second, it dominates the left side of the visible spectrum; indigo is nearly the only botanical dyestuff which provides a colorfast blue.
Indigo is a hot topic right now, hot in the dyeing community, and hot among scholars who work on dyes. I was recently at a conference on colors where half the papers were on indigo. I was at another on craft, where the only two papers on colors were also on that same blue dyestuff (one of those two was mine, since the green on Sterne’s page is half indigo). That seems odd. But I think it is because indigo uniquely combines two different qualities that make it such a popular dye. In the first place, it is exceptionally common in the natural world. Over two hundred plants yield enough indigo precursors that they could be plausibly cultivated for that dyestuff. And, in the second, it dominates the left side of the visible spectrum; indigo is nearly the only botanical dyestuff which provides a colorfast blue.
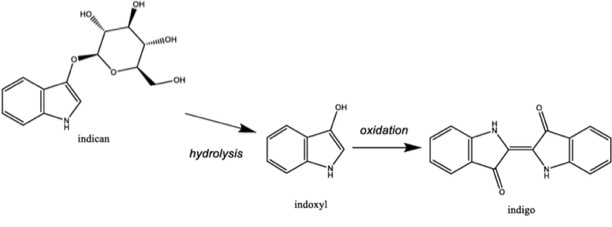
But indigo is also really, really weird. In the first place, we talk about plants as though they are “indigo,” but that’s not quite right. Brazilwood contains brazilin, the red dyestuff; lots of plants contain flavonoids, which are yellow. But not one of the hundreds of plants which yield indigo, plants in different genera and even families, contains indigo itself. Rather, each yields a chemically identical indigo precursor called “indican,” a glycoside which contains exactly half of the indigo dye molecule. A glycoside is just a glucose molecule attached to something else; plants use them for all sorts of things, including stashing away compounds for later use. Enzymes in the plant, usually stored somewhere that the glycosides are not, can click the glycosides together or apart. Clicking a compound to a sugar molecule stores both the sugar and the compound; breaking them apart releases both that compound, and a single sugar molecule—which can be used for fuel. That’s what indican is: glucose attached to a compound called indoxyl, which is the critical precursor for indigotin, the indigo color-molecule.
Here’s another way that indigo is strange. There are essentially two different ways to dye fabrics with indigo, but neither of these involves chemical bonds between the dye and the fabric. Indigo is a special kind of direct dye called a “vat” dye. Dyeing with it depends entirely on manipulating its solubility in water. Indoxyl, the indigo precursor, is highly soluble; it can therefore be carried in solution into the fibers and pores of materials like cotton, linen, silk, and wool. But in the presence of oxygen, two indoxyls fasten to each other to become indigotin, and indigotin won’t dissolve. So, having been carried into those pores or the gaps between fibers, and then exposed to the air, indigo (as indigotin) stays trapped there. It won’t wash out. That process is part of a field called physical chemistry, since, while there are some very weak chemical bonds which help stick indigotin to fabric fibers, it is mostly held mechanically. The special blue and white texture of denim results from “adsorption”; the cotton in denim is not dyed, but only holds some of the dye molecules for a while, while remaining white, itself.

Dyeing with indoxyl is the first method, and many different cultures have independently developed strategies for dyeing directly from the plants. But indoxyl can also be oxidized into indigotin and transformed to cakes of the insoluble pigment; stable and nearly pure, this is the commodity form of indigo. This labor was generally done right at the site of the indigo fields. When indigo became big business, basically the first color which was the object of plantation monoculture, it was rendered insoluble at an open-air digester called a vat. In the first open-air tub, the indoxyl was extracted from plant-matter and dissolved in water; in the second, it was oxidized, whence it becomes insoluble indigotin, sinks to the bottom of the vat, and is collected in cakes. As such, indigo was the most chemical of early modern dyes; each indigo plantation was in fact the outworks of a vast industrial chemical enterprise.
To dye with this, it needs to be rendered soluble, not by breaking it back into indoxyl, but by subtly transforming the indigotin molecule into a soluble compound called “leuco-indigo.” This is the way that most dyers work today: buying insoluble indigo in cakes and rendering it soluble through reduction. Reduction just means plucking away a few hydrogen molecules from the periphery of the indigotin compound; that’s enough to make it asymmetrical and give it enough polarity to dissolve in water. Then, the same process applies: the dyestuff is conducted into the fibers of the fabric, and exposed to oxygen, where it again becomes insoluble and stays trapped there.
So there are lots of different ways to get from the indican-bearing plants to the color-bearing indigotin, but they all wind up with the exact same chemical compound, more or less: the dark blue dye we call indigo. This is the first reason that so many papers, today, are on indigo: what we’re really discussing is lots of different relationships with different plants, which arrive at the same dyes.
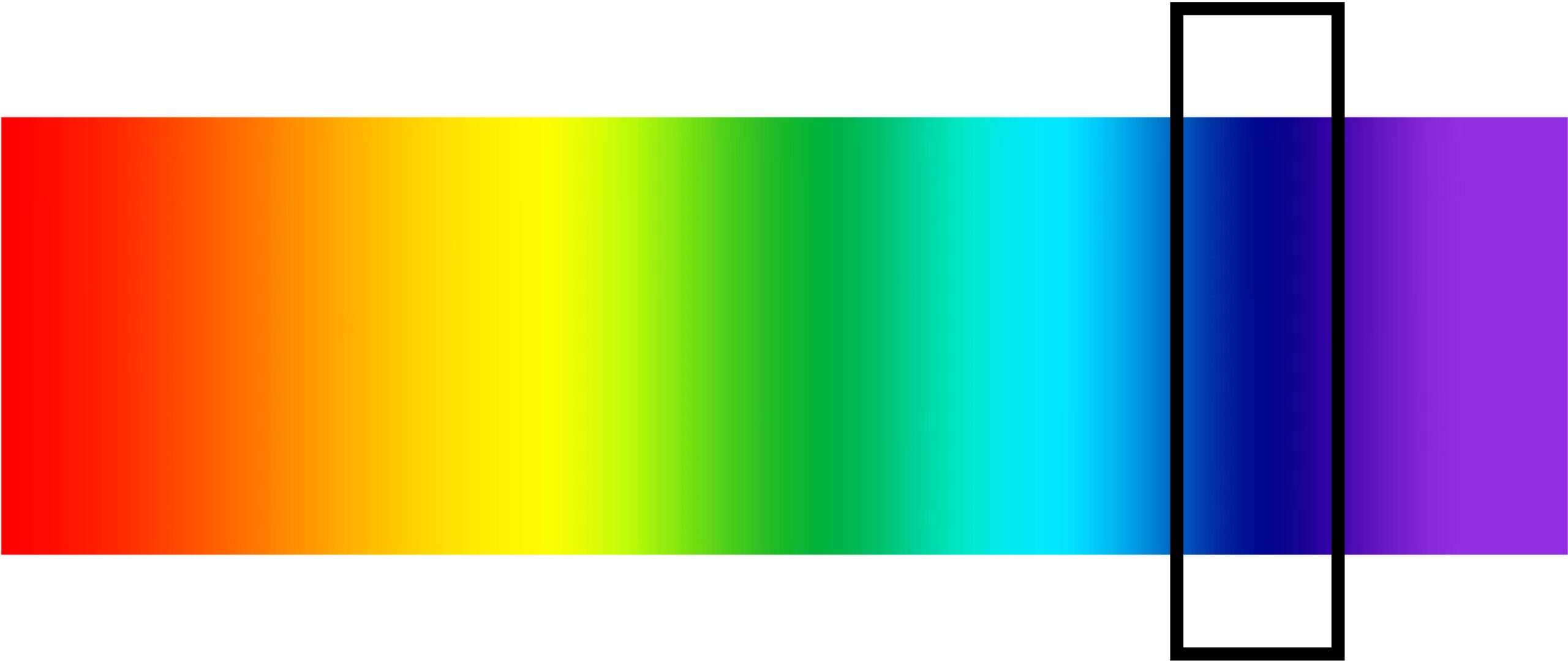
But there’s another reason indigo is so common as a natural dye, which has to do with its place on the spectrum. For virtually the whole spectrum right of green, the only tolerably colorfast natural dye is indigo—and indigo, because of its compact structure, is very colorfast indeed. So blue is indigo, so stable and pure, that Newton chose it to name the large band of hues between violet and cerulean. Our eyes aren’t especially attuned to differences of shades in that range; apart from the sea and the unclouded, daytime sky (which are admittedly vast), not much of nature is blue, so we haven’t evolved much sensitivity there. But when Newton saw how broad that visible band was, he felt compelled to divide it into two categories. To “blue” therefore, he added “indigo”: giving us the seven-color spectrum sometimes reduced, acrostically, to ROYGBIV.
Because its manufacture depends upon making the soluble insoluble, strange things have been reported of indigo. Cow’s milk, it has been said, is stained blue, when cattle are allowed to feed in indigo fields, and caterpillars feasting on indigo leaves have been said, likewise, to become blue from the inside out. Among the strangest reports is this: it was said that an egg, left near an indigo dyeworks, would become blue– not just the shell, but the yolk and white. Michal Taussig, in his lyrical thought-piece on indigo manufacture, makes a lot of this; and I must say that it is hard to balance the curious delight of discovering such a strange secret with what we know of the horrors of backbreaking plantation monoculture and the subtle chemical influence that the dye exerts—with the implication that it subtly stains everything it touches.
 Of course, it’s hard to read something like that without wondering if it’s actually true. I’ve got eggs; I’ve got indigotin from Indigofera suffruticosa (Guatemala indigo, one of the plantation varietals). And I’ve got time and reducing agents and alkalis and so on: enough to make an indigo vat. Future research will have to determine whether it is possible to dye an egg from the inside-out by exposing it to indoxyl extracted directly from plants. But I can say from experience that indigotin reduced to leuco-indigo—the second method—appears (alas) incapable of dyeing more than the shell. The shell, though: that is dyed through– and becomes an astonishingly delicate and mottled blue.
Of course, it’s hard to read something like that without wondering if it’s actually true. I’ve got eggs; I’ve got indigotin from Indigofera suffruticosa (Guatemala indigo, one of the plantation varietals). And I’ve got time and reducing agents and alkalis and so on: enough to make an indigo vat. Future research will have to determine whether it is possible to dye an egg from the inside-out by exposing it to indoxyl extracted directly from plants. But I can say from experience that indigotin reduced to leuco-indigo—the second method—appears (alas) incapable of dyeing more than the shell. The shell, though: that is dyed through– and becomes an astonishingly delicate and mottled blue.
 I also had this idea that if cellulose in cotton was such a good vehicle for indigotin, and cellulose is the most prevalent substance in woody plants like trees, that I should be able to stain wood the same way. Here at the Motley Lab, we like to say that we get things wrong, but we get them wrong in an interesting way. It is spring, and spring is the season of birdhouses, so: what better way to paint a birdhouse than immersing it in soluble leuco-indigo? I was aiming for a good, dark, blue-jeans blue; what I got was a color like the palest cerulean, where the sky meets the horizon, or, let’s say, well-faded blue-jeans. Deeper colors will have to wait for future research.
I also had this idea that if cellulose in cotton was such a good vehicle for indigotin, and cellulose is the most prevalent substance in woody plants like trees, that I should be able to stain wood the same way. Here at the Motley Lab, we like to say that we get things wrong, but we get them wrong in an interesting way. It is spring, and spring is the season of birdhouses, so: what better way to paint a birdhouse than immersing it in soluble leuco-indigo? I was aiming for a good, dark, blue-jeans blue; what I got was a color like the palest cerulean, where the sky meets the horizon, or, let’s say, well-faded blue-jeans. Deeper colors will have to wait for future research.