When we think of paper, especially historical paper, we tend to think of white paper. We think of the fine white paper used for books, or for letters or manuscript notes or diaries or accounting– and lots of that paper was being made by the eighteenth century. But yet more paper was being used for other things, including printing of documents not designed to be saved– like newspapers– or for any purpose where a soft, cheap, flexible medium would do. So: for every sheet of paper used to print a novel or to write a letter, there were ten or more used for scribbling accounts, or enclosing things for shipping, or wrapping packages like sugar or fabrics or medicines, or oiling for windows, or lining pie-sheets, and so forth.

But that sense of most paper as being white is relatively recent. Paper in eighteenth-century Europe was overwhelmingly made from recycled linen rags. And, in the absence of effective chemical bleaches, to make the best paper, they needed the very cleanest ragstock. As you can probably imagine, that sort of linen, discarded as unfit for use, but still white, was also the rarest. So most of that linen went to make less desirable paper, like the kinds known as “brown” or “whitey-brown.” This was still useful for wrapping packages or preparing things for mailing, and so on, since it had all the strength and flexibility of paper. It just wasn’t as useful for writing. A further batch of mildly soiled paper could solve the problem in a different way– by being dyed or stained. Mid-range paper dyed with indigo, for instance, was commonly used to wrap products like sugar and flour; it also came into vogue as a medium for pastels. The artist Jonathan Richardson sketched endless self-portraits on blue paper. Sometimes he used discarded wrappers, and sometimes purchased new wrapping paper especially for that purpose.
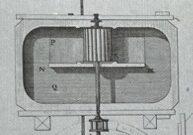
But that bluing– that also suggested another strategy, which came to characterize paper from Holland. Papermakers in Holland faced a problem of elevation, since that country is almost entirely at sea-level. The problem of power they overcame by developing a cylindrical machine that could be driven by wind. That machine is called a Hollander, and the cellulose of the paper in the Motley Emblem passed through one. But without lots of clean, relatively pure water, Dutch paper tended to be slightly yellow when compared with the creamy-white paper from the mountainous region of France.
Dutch papermakers therefore sometimes treated the rag-pulp for high-grade writing papers with a nearly-transparent tincture of indigo. That pale blue additive filled out the yellowish spectrum, returning something closer to white. In time, what began as a workaround to approach the cream-color of French paper wound up being desirable in its own right, akin even to a trademark. Dutch paper, soft and thick, was sought for its blueish cast.
This has some interesting side effects– one of which has started to create a problem for this historical reconstruction. Last summer, I worked with a Philly-based papermaker to prepare a few trial batches of linen-rag paper. We began with what we thought was raw linen cloth, painstakingly cutting it in to small squares, then boiling it with an alkali, running it through a Hollander, and making a series of trial sheets, just as the Dutch papermakers had done in preparing the paper that would wind up in Sterne’s book. I knew nothing, then, about optical brighteners, or that they would cling even to microscopic fibers through the whole process of cooking, macerating, and sheet formation.


But they did– and this makes that batch of paper useless for this project. Take, for instance, tests recently ran using the sort of ultraviolet instrument forensic detectives employ. We were hoping to find, or to rule out, the presence of a handful of organic pigments which are known to fluoresce in the visible range when illuminated with ultraviolet light. Those pigments appear not to be present. What is obvious, however, are those optical brighteners—have made the mock-up nearly useless.
Brighteners, in other words, now present an adulterant on the order of the adulterants which Dutch papermakers worked so hard to exclude. They are now nearly ubiquitous, embedded in raw cloth and included in nearly all detergents. And because the goal of this project is to make sheets accurate enough to fool the usual techniques for pigment-identification, our next steps, therefore, involve finding a cellulose source untreated with brighteners. Like the Dutch, we must acquire linen rags which are clean of adulterants which the eye can see, like good old-fashioned dirt. But, unlike them, we must also find ones which are free of invisible adulterants, including the chemicals we use to create the illusion of cleanliness.