Projects
Example projects from previous years are described below. As with all research, these projects evolve, as do faculty’s interests and needs.
-
Faculty: David Shreiber
Collaborators: Melitta Schachner (Cell Biology & Neuroscience),
Virginia Ayres (Michigan State University, Dept of Electrical &
Computing Engineering)Contributions from Undergraduates/Previous REU: The dramatic changes in the physico-chemical environment following injury largely dictate functional outcomes and regenerative potential. From the previous REU, 6 students have (1) optimized microfluidic systems for the long term cell culture and assays; (2) characterized stem cell viability in photo-crosslinkable collagen; (3) assayed the response of reactive astrocytes, which can severely impede regeneration, to different polymeric nanofiber scaffolds and substrates; (4) modulated the bioactivity of collagen with specific peptide sequences to control astrocyte adhesion and reactivity; and (5) evaluated the influence of encapsulated mesenchymal stem cells (MSCs) on the response of astrocytes and neurons to specific insults that are consistent with secondary injury following trauma. Several of these students have presented (or will present in the Fall) at conferences, and one is a co-author on a manuscript in preparation. All of those who have graduated from their home institutions have continued to graduate school for a MS or PhD. One was recently awarded an NSF-GRF.
Future Projects: Future projects will involve generating novel materials that dictate the phenotype of astrocytes, patterning the photo-active collagen with specific molecules to control the neural tissue regeneration and priming encapsulated MSCs to enhance their ability to modulate the response of neural cells (astrocytes, neurons, oligodendrocytes, and microglia) to a controlled injury environment. A patent has recently been filed for the photo-active collagen, and students will be encouraged to develop innovative approaches to utilizing this material.
-
Faculty: Charlie Roth
Collaborators: Tamara Minko (Pharmacy), David Devore (U.S. Army Institute for Surgical Research)Contributions from Undergraduates/Previous REU: Our laboratory has developed a novel lipid-polymer nanocomposite that overcomes both systemic and intracellular barriers to the delivery of antisense oligonucleotides and siRNAs. The 3 REU students who have worked on this project to date have (1) demonstrated protection of oligonucleotides in the presence of serum using a gel-shift assay; (2) optimized a tumor cell spheroid model for use in nanocomposite delivery studies; and (3) investigated the combination of chemotherapeutic drug and STAT3 siRNA in the tumor spheroid model. All three REU students have presented at national conferences, with one wining a Best Poster Award at ABRCMS.
Future Projects: REU students in the next couple of years will work on characterizing targeted versions of the delivery system in tumors (NIH-funded) and in unwanted nascent bone tissue (DoD). They will also work on peptide antibiotic delivery for wound healing, an area for which we have recently filed a patent that follows our initial filing for oligonucleotide delivery.
-

Faculty: Jeffrey Zahn, BME
Collaborators: David Shreiber, (BME), Hao Lin (MAE), and Jerry Shan (MAE)Contributions from Undergraduates/Previous REU: In electroporation, the cell membrane is transiently permeabilized when the cell exposed to a strong electric field. During permeabilization, biologically active molecules, such as DNA, RNA, and proteins, can enter the cell and change the cell’s genotype or phenotype.During the previous REU experience, students utilized a specially designed microdevice to address limitations of the electroporation method; namely, limited molecular transport efficiency, and excessive cell damage/death. To accomplish this goal, we hypothesized that temporally controlling the application of the permeabilizing electric field as a doubled-pulse using a microfluidic device could improve transfection efficiency while maintaining cellular viability. The first pulse is a high intensity electric field which initiates the opening of the membrane via electroporation, while the second lower field pulse is used for material transport into the cell. As a result of the REU experience one student is currently beginning his MS program while the other intends to apply to graduate school in the upcoming year. Other REU students worked on a separate project that involved development of a microdevice for isolation and measurement of inflammation mediators during cardiac bypass.
Future Projects: Future projects will monitor the changed in electrical impedance of cells undergoing electroporation, and correlate those changes to cellular permeability and viability. By ending the electric field pulse upon permeabilization, unnecessary high-strength field exposure will be limited, improving cell viability. A technology disclosure for this idea has recently been filed, and students will be encouraged to develop innovative applications for utilizing this technique.
-
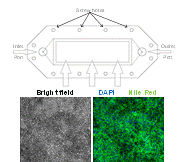 Faculty: François Berthiaume
Faculty: François Berthiaume
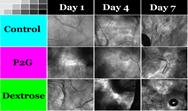
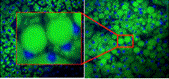
Collaborators: Martin Yarmush, (BME), James Guarrera (Columbia University)Contributions from Undergraduates/Previous REU:We aim to metabolically recondition livers that are discarded from the donor pool due to excessive fat content. To understand the effects of various environmental cues (eg hormones, amino acids, oxygen tension) on the mechanisms of defatting, we are developing a flow system that mimics the in vivo hydrodynamic environment of whole livers to observe the defatting in real-time. A prior team of undergraduate students has (1) used a 3D printer to generate various parallel plate flow designs similar to the one shown in the figure; (2) optimized culture methods to induce fat deposition in hepatoma cells (figure above shows intracellular fat stained with Nile red); (3) characterized the viability of fatty hepatoma cells in the flow chambers; (4) tested the effect of cocktails of agents that stimulate defatting and compared the rate of defatting with “static” cultures. These students graduated in the spring of 2012. One went on to graduate school. Two others went to industrial positions.
Future Projects: Future projects will involve optimizing the composition of the perfusing medium to achieve rapid defatting of the liver cells using the flow device, as well as a mathematical model of the liver biochemical network to determine the principal metabolic pathways to be regulated. The composition of the solution would be patentable and we will seek IP protection. Furthermore, the results from these studies will be directly translated to small and large animal studies (with our collaborators at Columbia University Medical Center). This information would then be used to justify human studies.
-
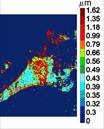 Faculty: Nada Boustany, BME
Faculty: Nada Boustany, BME
Collaborators : Eileen White, (Cancer Institute of New Jersey), Bonnie Firestein (Cell Biology & Neuroscience), Dimitris Metaxas (Computer Science)Contributions from Undergraduates/Previous REU: Fundamental biological processes, such as programmed cell death, differentiation, and division, involve time-dependent dynamic alterations in the morphology and subcellular organization of sub-micron sized organelles. As in any structure-function relationship, these changes are controlled by important molecular pathways, and must be quantified objectively to gain a more complete understanding of cellular function. We are developing a novel non-invasive approach to analyze the size, orientation, and distribution of subcellular structures within a living cell that is based on optical Fourier processing with Gabor filters. The advantages of this method are: (1) it is “label-free”; (2) it is highly sensitive to nanoscale changes in object structure; and (3) it can be implemented by observing large fields of view, thus allowing the rapid analysis of several hundred cells at a time. A microscope prototype has already been developed under an NSF-IDBR award. Over the past 3 years this interdisciplinary project has provided opportunities for 9 undergraduates to be trained in instrument development and design, optics and imaging, cell biology, computational methods and hardware automation. We aim to advance the project in three areas: 1) improve instrument development towards a more automated, robust, and user-friendly platform, 2) expand the applications of this technique by investigating neuronal process formation, collagen fiber formation, while continuing our current project to study the role of mitochondrial fission and fusion in apoptosis resistance, an important checkpoint for tumor growth, 3) devise new computational methods for image analysis that are tailored to the novel dynamic cell data generated by this microscopy technique.
Future Projects: These exciting opportunities will continue to be available in the future with additional cell biology (neurons) and biochemistry-based (collagen) projects. Combining biological experimental design and hypothesis testing with technology development is the underlying strength of this REU project. Productive undergraduates will likely contribute to innovative methodologies and participate in discovery-based research with potential for significant contributions to technology patents and original publications.
-

Faculty: Mark Pierce, Rutgers BME
Collaborators : Francois Berthiaume, (Rutgers BME), Darren Roblyer (Boston University)Contributions from Undergraduates / Previous REU: This project aims to build and test an optical imaging system which can quantify the concentrations of different components of biological tissue. By measuring the diffuse reflectance of light at different spatial frequencies, an inverse model can be used to estimate the scattering and absorption coefficients of the tissue at each pixel in a 2-D image. By repeating this process under illumination at several different wavelengths, we can construct the scattering and absorption spectra, which can in turn be analyzed to determine the concentrations of tissue chromophores including oxyhemoglobin, deoxyhemoglobin, lipids, and water. Key components of the imaging system are hardware (LED’s for illumination, DMD’s for pattern projection, CCD cameras for image capture…) and Matlab software to integrate pattern projection frequency, image acquisition, and image processing and model-based analysis. This project involves hardware and software design and evaluation, as well as development of robust standards for calibration and testing. Previous students assembled and tested hardware, characterized optical imaging performance (resolution, field of view,…), designed and fabricated optical phantoms to allow system calibration, and analyzed measurement data from biological systems.
Future Projects: Once this system is fully characterized, we plan to evaluate its utility in monitoring liver cells and tissues undergoing a novel defatting process by monitoring lipid concentration over time The underlying technology can also be applied to other biomedical applications which require continuous, non-invasive monitoring of metabolic and physiologic parameters.
-
Faculty: Joseph Freeman, BME
Collaborators: Mike Dunn, Dept of Orthopaedics, Francois Berthiaume, BMEContributions from Undergraduate/Previous REU: Proliferative Therapy, or Prolotherapy, works by causing damage to strained tissue, primarily through osmotic shock, and initiating the wound healing cascade to promote the growth of new tissue to the area. Many studies have looked into the effectiveness of the therapy to increase tissue strength in vivo, but none have studied the effects of prolotherapy on the cells at the site of injection. A previous undergraduate (1) designed a 3D alginate system to test the effect of prolotherapy on ligament fibroblasts, (2) tested the effect of 2 different prolotherapy solutions on fibroblast viability, (3) collagen production, and (4) non-collagenous protein production. The work from this study will be presented in an upcoming manuscript, and the student will be a co-author.
Future Projects: Future projects will involve studying the effect of prolotherapy on cell types that are involved in the wound healing cascade because this therapy works by forcing the wound healing cascade to act at the site of injury. The cell types to be investigated include macrophages and neutrophils.
-
Faculty: Ronke Olabisi
Collaborators: Prabhas Moghe, BME, Mike Dunn, UMDNJ Dept of Orthopaedics, Jeff Zahn, BMEContributions from Undergraduates/Previous REU: Mesenchymal stem cells (MSCs) immunoprotected by encapsulation within polyethylene glycol (PEG)microsperes can be delivered via needle injection to sites requiring bone repair. Although the lab at Rutgers is just starting, previous undergraduate mentorship involved REU and other undergraduate research programs. Students (1) characterized morphological changes within orthopedic tissues following limb lengthening; (2) evaluated the response of MSCs to microencapsulation parameters; and (3) optimized techniques to assay and quantify viability of permanently entrapped cells. Two students presented at conferences, and one student was an author on a published paper.
Future projects: REU students will assist in development of microfluidic systems to standardize MSC microencapsulation and patterning PEG hydrogels with biomineralization proteins in order to achieve spatial control of mineralization.
-
Faculty: Martin Yarmush, BME
Collaborators: Yannis Androulakis (BME), Ramsey Foty (Dept Surgery, UMDNJ)Contributions from Undergraduates/Previous REU: Metabolic engineering principles can be integrated within cell culture regimes to control a plethora of cell responses. For example, four previous REU students have used these principles to optimize vascularization in pancreatic islet cells in order to improve islet transplant efficiency by (1) computational data mining to identify key regulatory VEGF inducing proteins; (2) evaluating insulin production in hypoxic cells; (3) metabolic supplementation and metabolic flux analysis to improve pancreatic cell VEGF secretion; (4) multifactorial experimental design to reduce sub-cellular lipid distribution in hepatocytes. These students have presented their data at local or national conferences and are currently working towards completion of graduate degrees or are in the process of applying to graduate school.
Future Projects: Future projects, ultilizing a combination of computational multifactorial design and flux analysis, will develop improved media configurations for cell specific maturation and functional augmentation. These media configurations will be evaluated in both static culture and in simulated organ perfusion conditions. Student will be exposed to a variety of bioengineering techniques including computation, biochemical assays, microscopy and flow chamber device design and optimization.