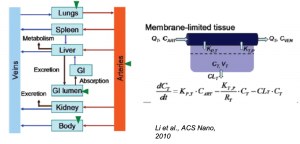
PBPK Modeling of Nanomedicines
There is great interest in the deployment of nanoparticle formulations of existing and next-generation drugs to improve their solubility, extend their circulation time, and thus increase their bioavailability. While a number of nanomedicines successfully prolong circulation time, the design rules for tailoring the biodistribution to specific applications remain rudimentary, although some progress is being made. Physiologically-based pharmacokinetic (PBPK) models are utilized to characterize the distribution of drugs in plasma and tissues and serve as a means to design alternative dosing schedules and translation among species and from animals to humans. The PBPK models typically employed to parameterize drug distribution need to be adapted to the physical mechanisms of nanoparticles, which may be scavenged by immune cells, are limited in their vascular permeability, and may not equilibrate in the manner of small molecule drugs. We are developing advanced nano-PBPK models that reflect the transport mechanisms by which nanoparticles traverse tissue and cellular barriers and using these to develop quantitative relationships between physical attributes of nanoparticles and their distribution behavior.