
Dr. Yong Mao is currently a Research Professor and the lead biologist in the Laboratory for Biomaterials Science. She joined Rutgers in 2013, having developed both academic and industrial research experience. Her research interests include cell-biomaterial interaction, extracellular matrix/synthetic hybrid bioactive scaffolds, stem cell technology, infection resistant biomaterials and wound healing. She has established and led multiple industrial collaborations. She also enjoys mentoring undergraduate students and developing their independent research capabilities. Dr. Mao directs the biological laboratory and microbiology laboratory at the LBR and leads the collaborations with internal and external research groups.
RESEARCH SUMMARY
Gelatin-based hydrogel for tissue engineering
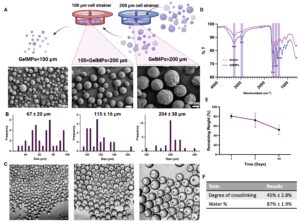
Gelatin and modified gelatin, such as methacrylated gelatin (GelMA), are versatile extracellular matrix (ECM)-derived macromolecules widely used as cell delivery carriers for tissue engineering and wound healing applications. Unlike the common encapsulation of cells for delivery, we have developed gelatin and GelMA microparticles, including microspheres of various sizes and microparticles generated by cryomilling. Our studies have demonstrated that cells exhibit better viability, proliferation, and functionality when cultured on the surface of microparticles compared to when they are encapsulated in hydrogels. Additionally, we have successfully used GelMA microspheres to create bovine adipose tissue. Currently, we are developing sophisticated microparticles with a multi-tier design to combine effective drug delivery and cell delivery for tissue generation.
Bioactive ECM-based polymeric scaffolds
Biodegradable polymeric scaffolds have gained tremendous interest in tissue regeneration in recent years due to their tunability in mechanical, structural and degradable properties. However, the lack of biological activity limits their integration with host tissues. To improve the biological activity of polymeric scaffolds for tissue-specific regeneration, we harness the biological cues from cell/tissue specific extracellular matrix (ECM) and combine them with polymeric scaffolds. By culturing lineage-specific cells on polymer scaffolds, followed by decellularization, hybrid scaffolds containing cell type specific ECM have been fabricated. These hybrid scaffolds guide the differentiation of human mesenchymal stem cells. Our research goal is to optimize the architecture of scaffolds, the biological activity of ECM and the presence of signaling peptides to support tissue regeneration for regenerative medicine and applications such as Cultivated meat.

In vitro expansion of primary cells for cell-based therapies
For many cell-based therapies, a large number of primary human cells are needed. For example, autologous chondrocyte implantation demands significant amplification of patients’ own chondrocytes to repair cartilage damage. However, the expansion of primary cells on tissue culture polystyrene results in dedifferentiation (loss of original cell phenotypes) of cells. We are exploring in vitro cell culture substrates to support cell expansion while minimizing cell dedifferentiation. Currently, modulating the cell adhesiveness to substrates and incorporating cell-type specific ECM components to substrates have demonstrated favorable effects on in vitro expansion of primary cells. We continue to explore the in vitro culturing conditions for human primary cells for therapeutic purposes.

Antimicrobial and anti-fibrotic activity of biological materials
Bacterial colonization in the wound environment or on implantable biomaterials leads to infection, which can result in more severe pathologies and failure of the implant. By characterizing the anti-bacterial activity of synthetic polymeric materials, we are able to facilitate the design and fabrication of infection-resistant biomaterials by chemists and material scientists.
Amniotic membranes (AM) have been used in wound treatment since ancient times. Recent years, processed AM have been developed into commercially available advanced biological wound dressing for treating chronic wounds. We are interested in understanding the properties and biological activities of AM. The antimicrobial peptides and exosomes (nano-sized vesicles) secreted by AM are our focuses in understanding the source and mechanism of antibacterial and anti-fibrotic activity of AM.
Publications
-
Human mesenchymal stem cells on sized optimized gelatin microparticles show enhanced wound healing activities. Gels 10 (2): 97, Ozhava D, Bektas C, Lee K, Jackson A, Mao Y*, 2024
https://doi.org/10.3390/gels10020097 -
Human Blood Protein Cocktail Decreases Burn Wound Expansion and Bacterial Growth. Journal of Biological Regulators and Homeostatic Agents 38(5):3815-3827, Reddy SV, Kumar S, Manjunath G, Kungumaraj HJ, Susin-Pires I, O’Boyle Q, Mao Y, Palmer AF, Berthiaume F, 2024
https://www.biolifesas.org/EN/10.23812/j.biol.regul.homeost.agents.20243805.301 -
Enhancing Antimicrobial Activity and Reducing Cytotoxicity of Silver Nanoparticles through Stabilization on Gelatin Nanoparticles. Nanomedicine 19 (3): 199-211 , Ozhava D, Winkler P, Mao Y*, 2024
https://pubmed.ncbi.nlm.nih.gov/38271055/ -
Hydrogel Microparticles for Bone Regeneration. Gels 10 (1): 28, Bektas C and Mao Y, 2023
https://doi.org/10.3390/gels10010028 -
Placental-Derived Biomaterials and Their Application to Wound Healing: A Review. Bioengineering 10:829, Protzman NM, Mao Y, Long D, Sivalenka R, Gosiewska A, Hariri RJ, Brigido SA, 2023
https://pubmed.ncbi.nlm.nih.gov/37508856/ -
A tri-layer decellularized, dehydrated human amniotic membrane scaffold supports the cellular functions of human tenocytes in vitro. Journal of Materials Science: Materials in Medicine 34:37, Mao Y, John N, Protzman NM, Long D, Sivalenka R, Azimi S, Mirabile B, Pouliot R, Gosiewska A, Hariri RJ, Brigido SA, 2023
https://pubmed.ncbi.nlm.nih.gov/37486403/ -
Diblock and triblock tyrosine-derived polymeric surfactants as cell membrane modifiers. Journal of colloid and interface science 644:264-274, Lima MRN, Le KP, Chakhalian D, Mao Y, Kohn J, Devore DI. , 2023
https://pubmed.ncbi.nlm.nih.gov/37120875/ -
Bovine Fibroblast-Derived Extracellular Matrix Promotes the Growth and Preserves the Stemness of Bovine Stromal Cells during In Vitro Expansion. J. Funct. Biomater. 14:218-232. , Lee K, Jackson A, John N, Zhang R, Ozhava D, Bhatia M, Mao Y*. , 2023
https://doi.org/10.3390/jfb14040218 -
An in-vitro comparison of human corneal epithelial cell activity and inflammatory response on differently designed ocular amniotic membranes and clinical case study. Journal of Biomedical Materials Research Part B Applied Biomaterials 111:684–700, Mao Y. Protzman NM, John N, Kuehn A, Long D, Sivalenka R, Junka R, Shah AU, Gosiewska A, Hariri RJ, Brigido SA, 2023
https://pubmed.ncbi.nlm.nih.gov/36370413/ -
Sustainable Cell Sources for Cultivated Meat. Journal of Biomedical Research and Environmental Sciences 3(11): 1382-1388, Ozhava D, Bhatia M, Freeman J, Mao Y*. , 2022
https://www.jelsciences.com/articles/jbres1607.pdf -
A decellularized flowable placental connective tissue matrix promotes cellular functions of human tenocytes in vitro. Journal of Experimental Orthopedics 9 (1): 1-16, Mao Y. John N, Protzman NM, Kuehn A, Long D, Sivalenka R, Gosiewska A, Hariri RJ, Brigido SA, 2022
https://pubmed.ncbi.nlm.nih.gov/35849201/ -
Decellularized and Dehydrated Human Amniotic Membrane in Wound Management: Modulation of Macrophage Differentiation and Activation. Journal of Biology and Medicine 6 (1), 10-20, Protzman NM, Mao Y, Sivalenka R, Gosiewska A, Hariri RJ, Brigido SA, 2022
https://www.biolscigroup.us/articles/JBM-6-130.pdf -
Self-Assembled GelMA microparticle-based, organoid-like structures for tooth germ regeneration. Bioengineering 9(5), 215, Bektas C, Zhang W, Mao Y, Wu X, Kohn J and Yelick PC, 2022
https://pubmed.ncbi.nlm.nih.gov/35621493/ -
Exosomes Secreted from Amniotic Membrane Contribute to Its Anti-Fibrotic Activity. Int J Mol Sci , Mao Y*, Jacob V, Singal A, Lei S., Park MS, Lima M, Li C. Dhall S, Sathyamoorthy M, Kokn J. , 2021
https://doi.org/10.3390/ijms22042055 -
Thymoquinone-Loaded Polymeric Films and Hydrogels for Bacterial Disinfection and Wound Healing. Biomedicines., Haq A, Kumar S, Mao Y, Berthiaume F, Michniak-Kohn B. , 2020
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7600314/ -
In Vitro Evaluation of Recombinant Bone Morphogenetic Protein-2 Bioactivity for Regenerative Medicine., Fung SL, Wu X, Maceren JP, Mao Y, Kohn J., 2019
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6761583/ -
Endogenous viable cells in lyopreserved amnion retain differentiation potential and anti-fibrotic activity in vitro. , Mao Y, Hoffman T, Dhall S, Singal A, Sathyamoorthy M, Danilkovitch A, Kohn J. , 2019
https://www.sciencedirect.com/science/article/pii/S174270611930412X -
Processed human amniotic fluid retains its antibacterial activity, Mao Y, Pierce J, Singh-Varma A, Boyer M, Kohn J, Reems JA. , 2019
https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-019-1812-8 -
Patterned electrical impulses delivered to Schwann cells by an artificial axon in the suspended-wire culture model, Merolli A, Mao Y, Voronin G, Steele J, Murthy NS, Kohn J, 2019
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6404504/ -
Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation, Mao Y, Block T, Singh-Varma A, Sheldrake A, Leeth R, Griffey S and Kohn J. , 2019
https://pubmed.ncbi.nlm.nih.gov/30528605/ -
“Ruffled border” formation on a CaP-free substrate: a first step towards osteoclast-recruiting bone-grafts materials able to re-establish bone turn-over, Merolli A, Fung S, Murthy NS, Pashuck, ET, Mao Y, Wu X, Steele J, Martin D, Moghe P, Bromage T, Kohn J, 2018
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5862932/ -
The Effect of Cryopreserved Human Placental Tissues on Biofilm Formation of Wound-Associated Pathogens., Mao Y, Singh-Varma A, Hoffman T, Dhall S, Danilkovitch A and Kohn J. , 2018
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5872089/ -
An Innovative Laboratory Procedure to Expand Chondrocytes with Reduced Dedifferentiation. , Mao Y, Hoffman T, Wu A, Kohn J. , 2018
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5871131/ -
Influence of the three-dimensional culture of human bone marrow mesenchymal stromal cells within a macroporous polysaccharides scaffold on Pannexin 1 and Pannexin 3, Guerrero J, Oliveira H, Aid R, Bareille R, Siadous R, Letourneur D, Mao Y, Kohn J, Amédée J, 2018
https://pubmed.ncbi.nlm.nih.gov/29222846/ -
Enhancement of plasma protein adsorption and osteogenesis of hMSCs by functionalized siloxane coatings for titanium implants, Martínez-Ibáñez M, Murthy NS, Mao Y, Suay J, Gurruchaga M, Goñi I, Kohn J, 2018
https://pubmed.ncbi.nlm.nih.gov/28544508/ -
Antimicrobial Peptides Secreted From Human Cryopreserved Viable Amniotic Membrane Contribute to its Antibacterial Activity, Mao Y, Hoffman T, Singh-Varma A, Duan-Arnold Y, Moorman M, Danilkovitch A, Kohn J, 2017
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5651856/ -
Cell type-specific extracellular matrix guided the differentiation of human mesenchymal stem cells in 3D polymeric scaffolds. , Mao Y, Hoffman T, Wu A, Goyal R, Kohn J., 2017
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5440495/ -
A suspended carbon fiber culture to model myelination by human Schwann cells, Merolli A, Mao Y, Kohn J. , 2017
https://pubmed.ncbi.nlm.nih.gov/28210970/ -
Optimization of Polymer-ECM Composite Scaffolds for Tissue Engineering: Effect of Cells and Culture Conditions on Polymeric Nanofiber Mats , Goyal R, Guvendiren M, Freeman O, Mao Y, Kohn J., 2017
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5371874/